Xutong Kuang
CMRxRecon: An open cardiac MRI dataset for the competition of accelerated image reconstruction
Sep 19, 2023



Abstract:Cardiac magnetic resonance imaging (CMR) has emerged as a valuable diagnostic tool for cardiac diseases. However, a limitation of CMR is its slow imaging speed, which causes patient discomfort and introduces artifacts in the images. There has been growing interest in deep learning-based CMR imaging algorithms that can reconstruct high-quality images from highly under-sampled k-space data. However, the development of deep learning methods requires large training datasets, which have not been publicly available for CMR. To address this gap, we released a dataset that includes multi-contrast, multi-view, multi-slice and multi-coil CMR imaging data from 300 subjects. Imaging studies include cardiac cine and mapping sequences. Manual segmentations of the myocardium and chambers of all the subjects are also provided within the dataset. Scripts of state-of-the-art reconstruction algorithms were also provided as a point of reference. Our aim is to facilitate the advancement of state-of-the-art CMR image reconstruction by introducing standardized evaluation criteria and making the dataset freely accessible to the research community. Researchers can access the dataset at https://www.synapse.org/#!Synapse:syn51471091/wiki/.
The Extreme Cardiac MRI Analysis Challenge under Respiratory Motion (CMRxMotion)
Oct 12, 2022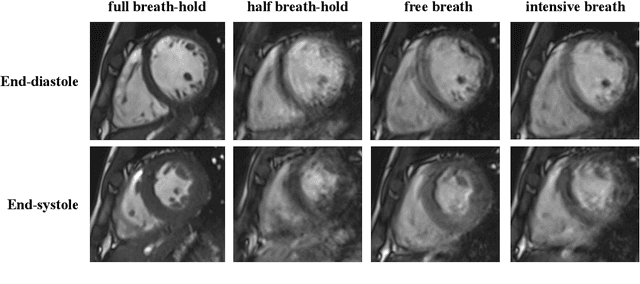
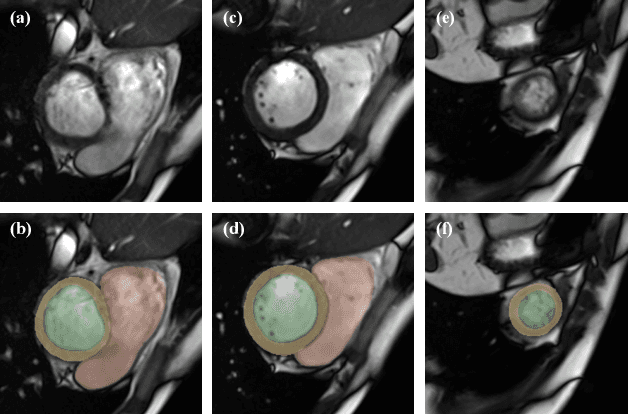

Abstract:The quality of cardiac magnetic resonance (CMR) imaging is susceptible to respiratory motion artifacts. The model robustness of automated segmentation techniques in face of real-world respiratory motion artifacts is unclear. This manuscript describes the design of extreme cardiac MRI analysis challenge under respiratory motion (CMRxMotion Challenge). The challenge aims to establish a public benchmark dataset to assess the effects of respiratory motion on image quality and examine the robustness of segmentation models. The challenge recruited 40 healthy volunteers to perform different breath-hold behaviors during one imaging visit, obtaining paired cine imaging with artifacts. Radiologists assessed the image quality and annotated the level of respiratory motion artifacts. For those images with diagnostic quality, radiologists further segmented the left ventricle, left ventricle myocardium and right ventricle. The images of training set (20 volunteers) along with the annotations are released to the challenge participants, to develop an automated image quality assessment model (Task 1) and an automated segmentation model (Task 2). The images of validation set (5 volunteers) are released to the challenge participants but the annotations are withheld for online evaluation of submitted predictions. Both the images and annotations of the test set (15 volunteers) were withheld and only used for offline evaluation of submitted containerized dockers. The image quality assessment task is quantitatively evaluated by the Cohen's kappa statistics and the segmentation task is evaluated by the Dice scores and Hausdorff distances.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge