Shengbo Gao
A Robust Ensemble Algorithm for Ischemic Stroke Lesion Segmentation: Generalizability and Clinical Utility Beyond the ISLES Challenge
Apr 03, 2024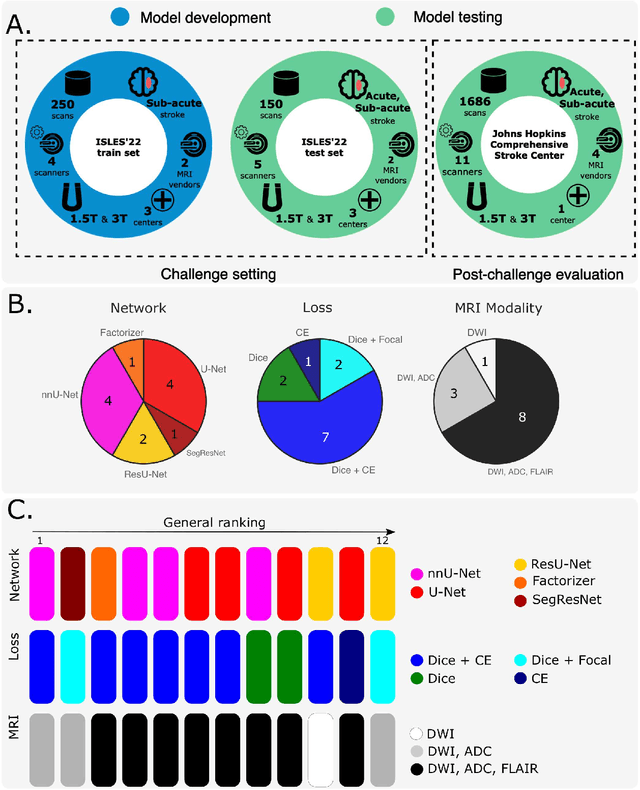
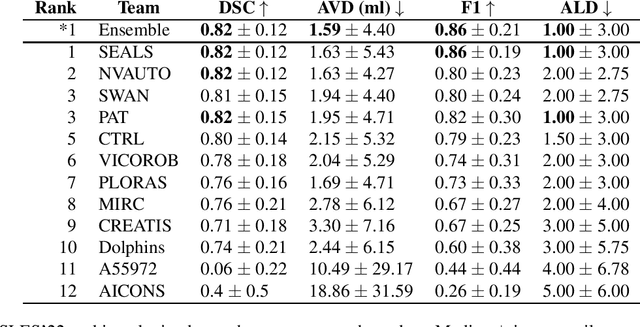
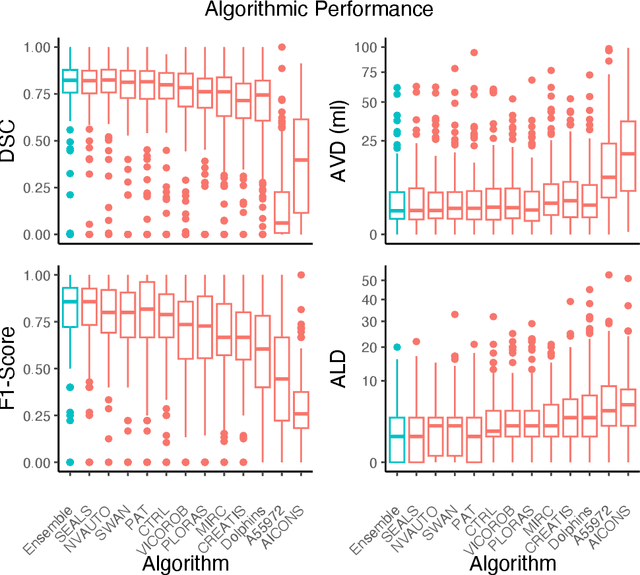
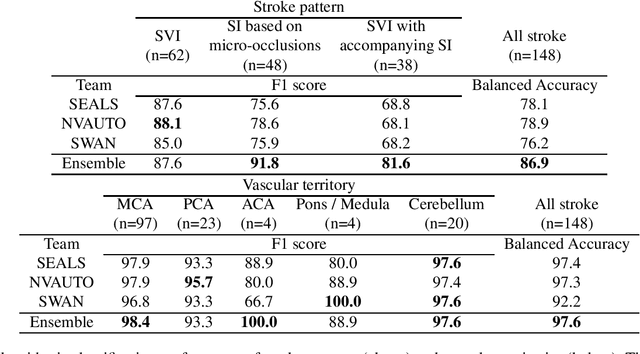
Abstract:Diffusion-weighted MRI (DWI) is essential for stroke diagnosis, treatment decisions, and prognosis. However, image and disease variability hinder the development of generalizable AI algorithms with clinical value. We address this gap by presenting a novel ensemble algorithm derived from the 2022 Ischemic Stroke Lesion Segmentation (ISLES) challenge. ISLES'22 provided 400 patient scans with ischemic stroke from various medical centers, facilitating the development of a wide range of cutting-edge segmentation algorithms by the research community. Through collaboration with leading teams, we combined top-performing algorithms into an ensemble model that overcomes the limitations of individual solutions. Our ensemble model achieved superior ischemic lesion detection and segmentation accuracy on our internal test set compared to individual algorithms. This accuracy generalized well across diverse image and disease variables. Furthermore, the model excelled in extracting clinical biomarkers. Notably, in a Turing-like test, neuroradiologists consistently preferred the algorithm's segmentations over manual expert efforts, highlighting increased comprehensiveness and precision. Validation using a real-world external dataset (N=1686) confirmed the model's generalizability. The algorithm's outputs also demonstrated strong correlations with clinical scores (admission NIHSS and 90-day mRS) on par with or exceeding expert-derived results, underlining its clinical relevance. This study offers two key findings. First, we present an ensemble algorithm (https://github.com/Tabrisrei/ISLES22_Ensemble) that detects and segments ischemic stroke lesions on DWI across diverse scenarios on par with expert (neuro)radiologists. Second, we show the potential for biomedical challenge outputs to extend beyond the challenge's initial objectives, demonstrating their real-world clinical applicability.
Correlation-Aware Mutual Learning for Semi-supervised Medical Image Segmentation
Jul 12, 2023Abstract:Semi-supervised learning has become increasingly popular in medical image segmentation due to its ability to leverage large amounts of unlabeled data to extract additional information. However, most existing semi-supervised segmentation methods only focus on extracting information from unlabeled data, disregarding the potential of labeled data to further improve the performance of the model. In this paper, we propose a novel Correlation Aware Mutual Learning (CAML) framework that leverages labeled data to guide the extraction of information from unlabeled data. Our approach is based on a mutual learning strategy that incorporates two modules: the Cross-sample Mutual Attention Module (CMA) and the Omni-Correlation Consistency Module (OCC). The CMA module establishes dense cross-sample correlations among a group of samples, enabling the transfer of label prior knowledge to unlabeled data. The OCC module constructs omni-correlations between the unlabeled and labeled datasets and regularizes dual models by constraining the omni-correlation matrix of each sub-model to be consistent. Experiments on the Atrial Segmentation Challenge dataset demonstrate that our proposed approach outperforms state-of-the-art methods, highlighting the effectiveness of our framework in medical image segmentation tasks. The codes, pre-trained weights, and data are publicly available.
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
Parameter Decoupling Strategy for Semi-supervised 3D Left Atrium Segmentation
Sep 20, 2021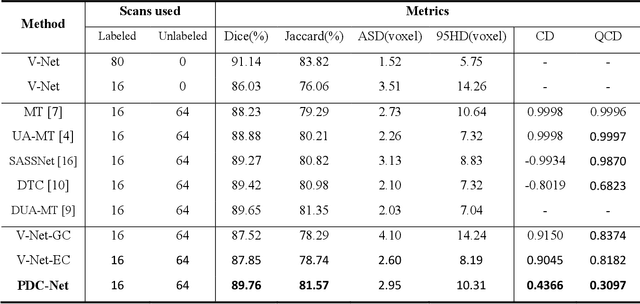
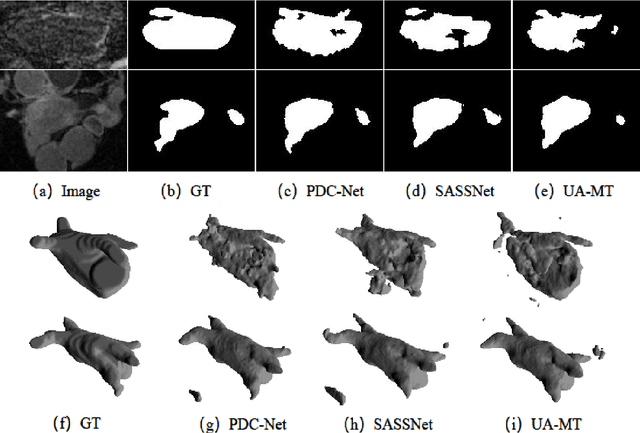
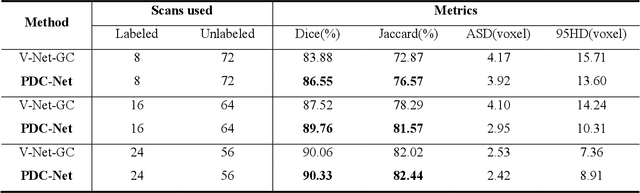
Abstract:Consistency training has proven to be an advanced semi-supervised framework and achieved promising results in medical image segmentation tasks through enforcing an invariance of the predictions over different views of the inputs. However, with the iterative updating of model parameters, the models would tend to reach a coupled state and eventually lose the ability to exploit unlabeled data. To address the issue, we present a novel semi-supervised segmentation model based on parameter decoupling strategy to encourage consistent predictions from diverse views. Specifically, we first adopt a two-branch network to simultaneously produce predictions for each image. During the training process, we decouple the two prediction branch parameters by quadratic cosine distance to construct different views in latent space. Based on this, the feature extractor is constrained to encourage the consistency of probability maps generated by classifiers under diversified features. In the overall training process, the parameters of feature extractor and classifiers are updated alternately by consistency regularization operation and decoupling operation to gradually improve the generalization performance of the model. Our method has achieved a competitive result over the state-of-the-art semi-supervised methods on the Atrial Segmentation Challenge dataset, demonstrating the effectiveness of our framework. Code is available at https://github.com/BX0903/PDC.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge