Philipp Berens
Hertie Institute for AI in Brain Health, University of Tübingen
Soft-CAM: Making black box models self-explainable for high-stakes decisions
May 23, 2025Abstract:Convolutional neural networks (CNNs) are widely used for high-stakes applications like medicine, often surpassing human performance. However, most explanation methods rely on post-hoc attribution, approximating the decision-making process of already trained black-box models. These methods are often sensitive, unreliable, and fail to reflect true model reasoning, limiting their trustworthiness in critical applications. In this work, we introduce SoftCAM, a straightforward yet effective approach that makes standard CNN architectures inherently interpretable. By removing the global average pooling layer and replacing the fully connected classification layer with a convolution-based class evidence layer, SoftCAM preserves spatial information and produces explicit class activation maps that form the basis of the model's predictions. Evaluated on three medical datasets, SoftCAM maintains classification performance while significantly improving both the qualitative and quantitative explanation compared to existing post-hoc methods. Our results demonstrate that CNNs can be inherently interpretable without compromising performance, advancing the development of self-explainable deep learning for high-stakes decision-making.
A Hybrid Fully Convolutional CNN-Transformer Model for Inherently Interpretable Medical Image Classification
Apr 11, 2025Abstract:In many medical imaging tasks, convolutional neural networks (CNNs) efficiently extract local features hierarchically. More recently, vision transformers (ViTs) have gained popularity, using self-attention mechanisms to capture global dependencies, but lacking the inherent spatial localization of convolutions. Therefore, hybrid models combining CNNs and ViTs have been developed to combine the strengths of both architectures. However, such hybrid CNN-ViT models are difficult to interpret, which hinders their application in medical imaging. In this work, we introduce an interpretable-by-design hybrid fully convolutional CNN-Transformer architecture for medical image classification. Unlike widely used post-hoc saliency methods for ViTs, our approach generates faithful and localized evidence maps that directly reflect the model's decision process. We evaluated our method on two medical image classification tasks using color fundus images. Our model not only achieves state-of-the-art predictive performance compared to both black-box and interpretable models but also provides class-specific sparse evidence maps in a single forward pass. The code is available at: https://anonymous.4open.science/r/Expl-CNN-Transformer/.
Learning Disease State from Noisy Ordinal Disease Progression Labels
Mar 13, 2025Abstract:Learning from noisy ordinal labels is a key challenge in medical imaging. In this work, we ask whether ordinal disease progression labels (better, worse, or stable) can be used to learn a representation allowing to classify disease state. For neovascular age-related macular degeneration (nAMD), we cast the problem of modeling disease progression between medical visits as a classification task with ordinal ranks. To enhance generalization, we tailor our model to the problem setting by (1) independent image encoding, (2) antisymmetric logit space equivariance, and (3) ordinal scale awareness. In addition, we address label noise by learning an uncertainty estimate for loss re-weighting. Our approach learns an interpretable disease representation enabling strong few-shot performance for the related task of nAMD activity classification from single images, despite being trained only on image pairs with ordinal disease progression labels.
Benchmarking Dependence Measures to Prevent Shortcut Learning in Medical Imaging
Jul 29, 2024



Abstract:Medical imaging cohorts are often confounded by factors such as acquisition devices, hospital sites, patient backgrounds, and many more. As a result, deep learning models tend to learn spurious correlations instead of causally related features, limiting their generalizability to new and unseen data. This problem can be addressed by minimizing dependence measures between intermediate representations of task-related and non-task-related variables. These measures include mutual information, distance correlation, and the performance of adversarial classifiers. Here, we benchmark such dependence measures for the task of preventing shortcut learning. We study a simplified setting using Morpho-MNIST and a medical imaging task with CheXpert chest radiographs. Our results provide insights into how to mitigate confounding factors in medical imaging.
This actually looks like that: Proto-BagNets for local and global interpretability-by-design
Jun 24, 2024Abstract:Interpretability is a key requirement for the use of machine learning models in high-stakes applications, including medical diagnosis. Explaining black-box models mostly relies on post-hoc methods that do not faithfully reflect the model's behavior. As a remedy, prototype-based networks have been proposed, but their interpretability is limited as they have been shown to provide coarse, unreliable, and imprecise explanations. In this work, we introduce Proto-BagNets, an interpretable-by-design prototype-based model that combines the advantages of bag-of-local feature models and prototype learning to provide meaningful, coherent, and relevant prototypical parts needed for accurate and interpretable image classification tasks. We evaluated the Proto-BagNet for drusen detection on publicly available retinal OCT data. The Proto-BagNet performed comparably to the state-of-the-art interpretable and non-interpretable models while providing faithful, accurate, and clinically meaningful local and global explanations. The code is available at https://github.com/kdjoumessi/Proto-BagNets.
Benchmarking Retinal Blood Vessel Segmentation Models for Cross-Dataset and Cross-Disease Generalization
Jun 21, 2024



Abstract:Retinal blood vessel segmentation can extract clinically relevant information from fundus images. As manual tracing is cumbersome, algorithms based on Convolution Neural Networks have been developed. Such studies have used small publicly available datasets for training and measuring performance, running the risk of overfitting. Here, we provide a rigorous benchmark for various architectural and training choices commonly used in the literature on the largest dataset published to date. We train and evaluate five published models on the publicly available FIVES fundus image dataset, which exceeds previous ones in size and quality and which contains also images from common ophthalmological conditions (diabetic retinopathy, age-related macular degeneration, glaucoma). We compare the performance of different model architectures across different loss functions, levels of image qualitiy and ophthalmological conditions and assess their ability to perform well in the face of disease-induced domain shifts. Given sufficient training data, basic architectures such as U-Net perform just as well as more advanced ones, and transfer across disease-induced domain shifts typically works well for most architectures. However, we find that image quality is a key factor determining segmentation outcomes. When optimizing for segmentation performance, investing into a well curated dataset to train a standard architecture yields better results than tuning a sophisticated architecture on a smaller dataset or one with lower image quality. We distilled the utility of architectural advances in terms of their clinical relevance therefore providing practical guidance for model choices depending on the circumstances of the clinical setting
Estimating Causal Effects with Double Machine Learning -- A Method Evaluation
Mar 21, 2024Abstract:The estimation of causal effects with observational data continues to be a very active research area. In recent years, researchers have developed new frameworks which use machine learning to relax classical assumptions necessary for the estimation of causal effects. In this paper, we review one of the most prominent methods - "double/debiased machine learning" (DML) - and empirically evaluate it by comparing its performance on simulated data relative to more traditional statistical methods, before applying it to real-world data. Our findings indicate that the application of a suitably flexible machine learning algorithm within DML improves the adjustment for various nonlinear confounding relationships. This advantage enables a departure from traditional functional form assumptions typically necessary in causal effect estimation. However, we demonstrate that the method continues to critically depend on standard assumptions about causal structure and identification. When estimating the effects of air pollution on housing prices in our application, we find that DML estimates are consistently larger than estimates of less flexible methods. From our overall results, we provide actionable recommendations for specific choices researchers must make when applying DML in practice.
Disentangling representations of retinal images with generative models
Feb 29, 2024



Abstract:Retinal fundus images play a crucial role in the early detection of eye diseases and, using deep learning approaches, recent studies have even demonstrated their potential for detecting cardiovascular risk factors and neurological disorders. However, the impact of technical factors on these images can pose challenges for reliable AI applications in ophthalmology. For example, large fundus cohorts are often confounded by factors like camera type, image quality or illumination level, bearing the risk of learning shortcuts rather than the causal relationships behind the image generation process. Here, we introduce a novel population model for retinal fundus images that effectively disentangles patient attributes from camera effects, thus enabling controllable and highly realistic image generation. To achieve this, we propose a novel disentanglement loss based on distance correlation. Through qualitative and quantitative analyses, we demonstrate the effectiveness of this novel loss function in disentangling the learned subspaces. Our results show that our model provides a new perspective on the complex relationship between patient attributes and technical confounders in retinal fundus image generation.
Self-supervised Visualisation of Medical Image Datasets
Feb 22, 2024



Abstract:Self-supervised learning methods based on data augmentations, such as SimCLR, BYOL, or DINO, allow obtaining semantically meaningful representations of image datasets and are widely used prior to supervised fine-tuning. A recent self-supervised learning method, $t$-SimCNE, uses contrastive learning to directly train a 2D representation suitable for visualisation. When applied to natural image datasets, $t$-SimCNE yields 2D visualisations with semantically meaningful clusters. In this work, we used $t$-SimCNE to visualise medical image datasets, including examples from dermatology, histology, and blood microscopy. We found that increasing the set of data augmentations to include arbitrary rotations improved the results in terms of class separability, compared to data augmentations used for natural images. Our 2D representations show medically relevant structures and can be used to aid data exploration and annotation, improving on common approaches for data visualisation.
Diffusion Tempering Improves Parameter Estimation with Probabilistic Integrators for Ordinary Differential Equations
Feb 19, 2024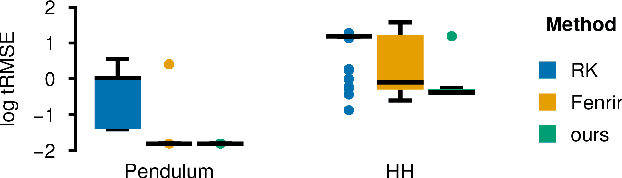
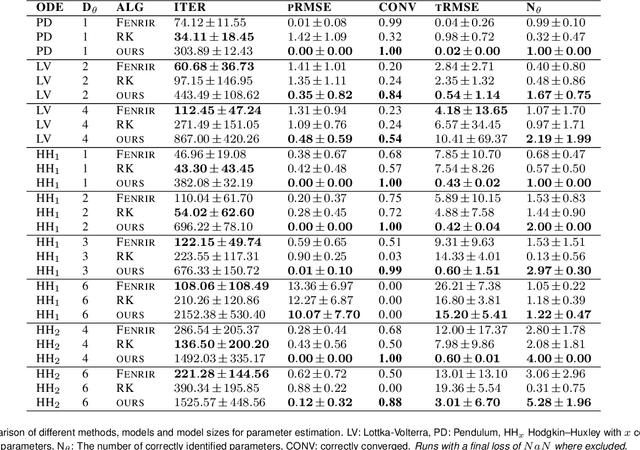
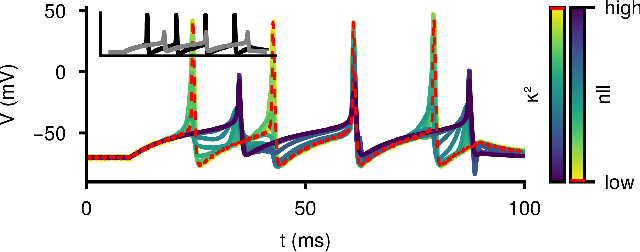

Abstract:Ordinary differential equations (ODEs) are widely used to describe dynamical systems in science, but identifying parameters that explain experimental measurements is challenging. In particular, although ODEs are differentiable and would allow for gradient-based parameter optimization, the nonlinear dynamics of ODEs often lead to many local minima and extreme sensitivity to initial conditions. We therefore propose diffusion tempering, a novel regularization technique for probabilistic numerical methods which improves convergence of gradient-based parameter optimization in ODEs. By iteratively reducing a noise parameter of the probabilistic integrator, the proposed method converges more reliably to the true parameters. We demonstrate that our method is effective for dynamical systems of different complexity and show that it obtains reliable parameter estimates for a Hodgkin-Huxley model with a practically relevant number of parameters.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge