Martin Faltys
Towards Foundation Models for Critical Care Time Series
Nov 25, 2024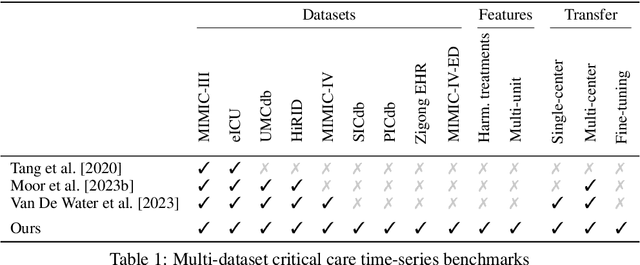
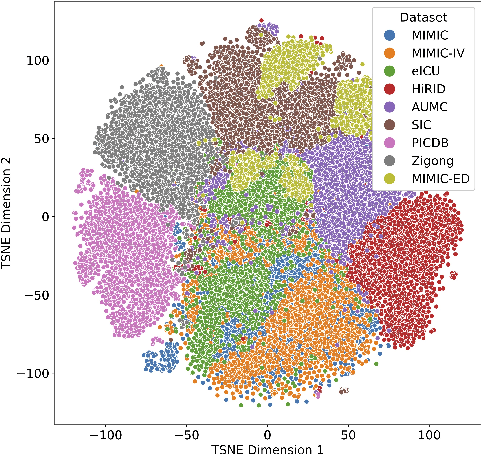
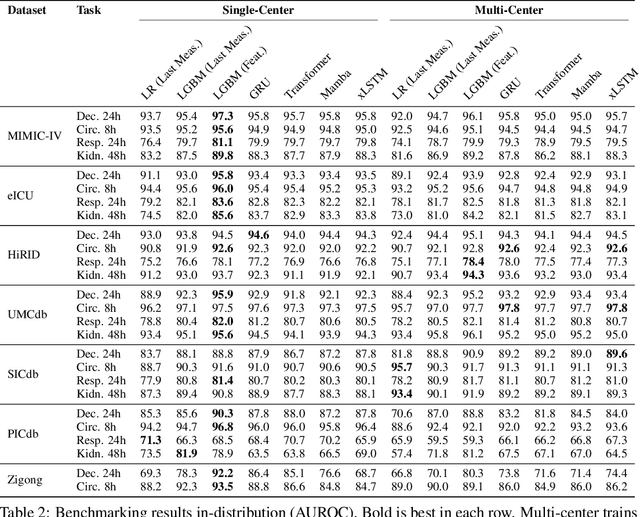
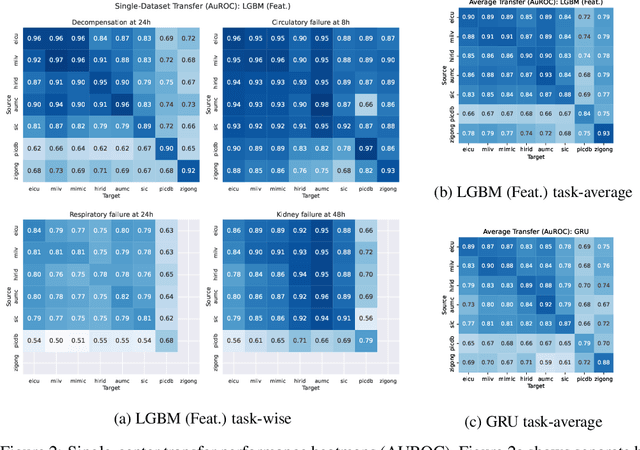
Abstract:Notable progress has been made in generalist medical large language models across various healthcare areas. However, large-scale modeling of in-hospital time series data - such as vital signs, lab results, and treatments in critical care - remains underexplored. Existing datasets are relatively small, but combining them can enhance patient diversity and improve model robustness. To effectively utilize these combined datasets for large-scale modeling, it is essential to address the distribution shifts caused by varying treatment policies, necessitating the harmonization of treatment variables across the different datasets. This work aims to establish a foundation for training large-scale multi-variate time series models on critical care data and to provide a benchmark for machine learning models in transfer learning across hospitals to study and address distribution shift challenges. We introduce a harmonized dataset for sequence modeling and transfer learning research, representing the first large-scale collection to include core treatment variables. Future plans involve expanding this dataset to support further advancements in transfer learning and the development of scalable, generalizable models for critical healthcare applications.
HiRID-ICU-Benchmark -- A Comprehensive Machine Learning Benchmark on High-resolution ICU Data
Nov 18, 2021
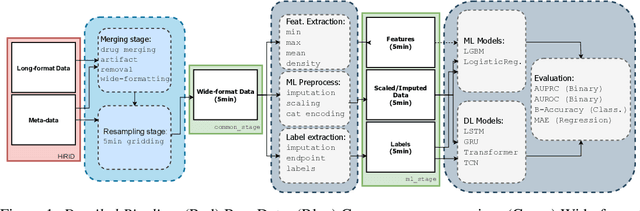
Abstract:The recent success of machine learning methods applied to time series collected from Intensive Care Units (ICU) exposes the lack of standardized machine learning benchmarks for developing and comparing such methods. While raw datasets, such as MIMIC-IV or eICU, can be freely accessed on Physionet, the choice of tasks and pre-processing is often chosen ad-hoc for each publication, limiting comparability across publications. In this work, we aim to improve this situation by providing a benchmark covering a large spectrum of ICU-related tasks. Using the HiRID dataset, we define multiple clinically relevant tasks developed in collaboration with clinicians. In addition, we provide a reproducible end-to-end pipeline to construct both data and labels. Finally, we provide an in-depth analysis of current state-of-the-art sequence modeling methods, highlighting some limitations of deep learning approaches for this type of data. With this benchmark, we hope to give the research community the possibility of a fair comparison of their work.
Early prediction of respiratory failure in the intensive care unit
May 12, 2021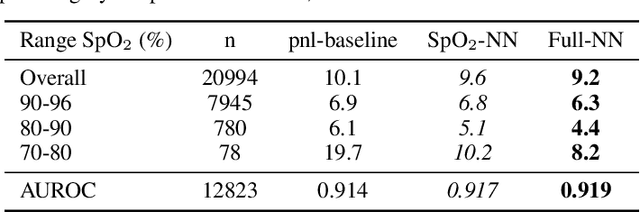
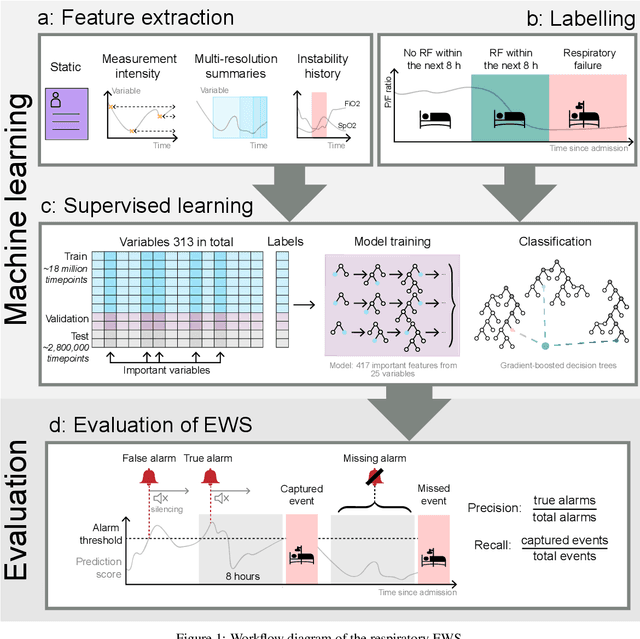
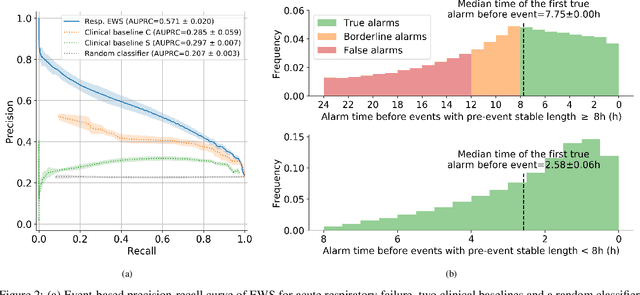
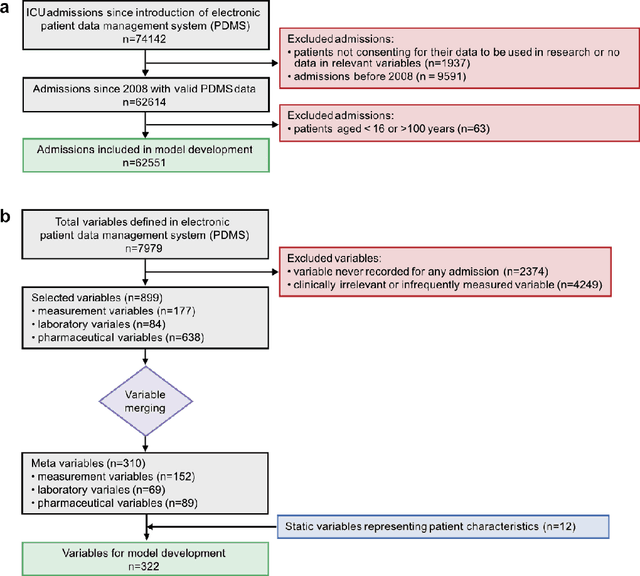
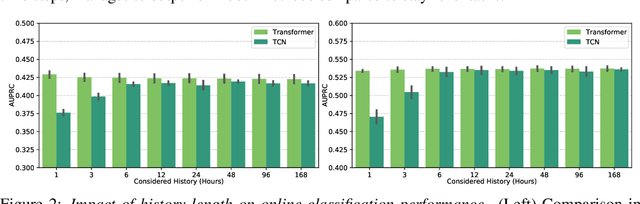
Abstract:The development of respiratory failure is common among patients in intensive care units (ICU). Large data quantities from ICU patient monitoring systems make timely and comprehensive analysis by clinicians difficult but are ideal for automatic processing by machine learning algorithms. Early prediction of respiratory system failure could alert clinicians to patients at risk of respiratory failure and allow for early patient reassessment and treatment adjustment. We propose an early warning system that predicts moderate/severe respiratory failure up to 8 hours in advance. Our system was trained on HiRID-II, a data-set containing more than 60,000 admissions to a tertiary care ICU. An alarm is typically triggered several hours before the beginning of respiratory failure. Our system outperforms a clinical baseline mimicking traditional clinical decision-making based on pulse-oximetric oxygen saturation and the fraction of inspired oxygen. To provide model introspection and diagnostics, we developed an easy-to-use web browser-based system to explore model input data and predictions visually.
WRSE -- a non-parametric weighted-resolution ensemble for predicting individual survival distributions in the ICU
Nov 02, 2020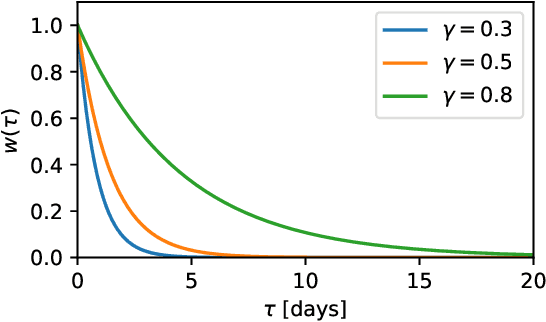
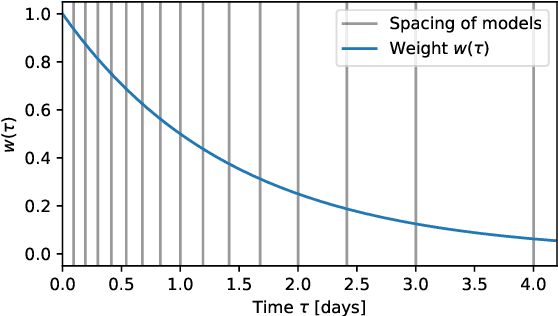

Abstract:Dynamic assessment of mortality risk in the intensive care unit (ICU) can be used to stratify patients, inform about treatment effectiveness or serve as part of an early-warning system. Static risk scoring systems, such as APACHE or SAPS, have recently been supplemented with data-driven approaches that track the dynamic mortality risk over time. Recent works have focused on enhancing the information delivered to clinicians even further by producing full survival distributions instead of point predictions or fixed horizon risks. In this work, we propose a non-parametric ensemble model, Weighted Resolution Survival Ensemble (WRSE), tailored to estimate such dynamic individual survival distributions. Inspired by the simplicity and robustness of ensemble methods, the proposed approach combines a set of binary classifiers spaced according to a decay function reflecting the relevance of short-term mortality predictions. Models and baselines are evaluated under weighted calibration and discrimination metrics for individual survival distributions which closely reflect the utility of a model in ICU practice. We show competitive results with state-of-the-art probabilistic models, while greatly reducing training time by factors of 2-9x.
Machine learning for early prediction of circulatory failure in the intensive care unit
Apr 19, 2019
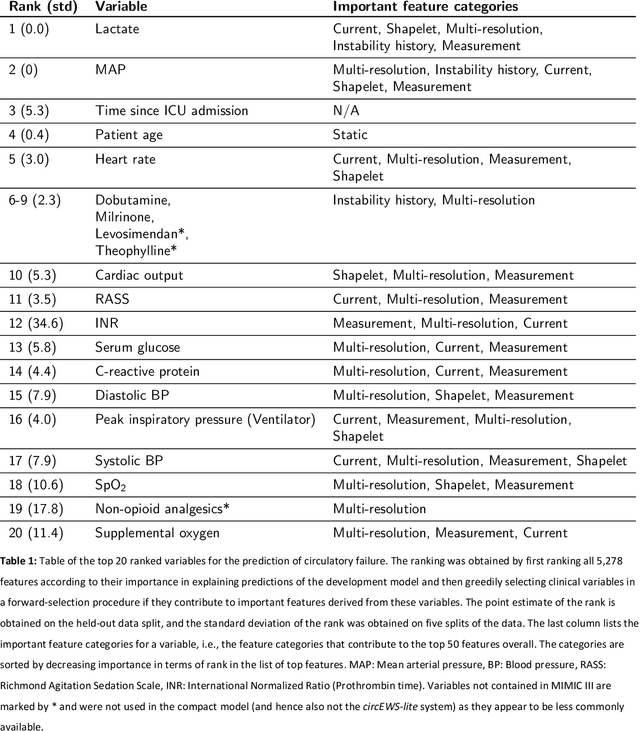

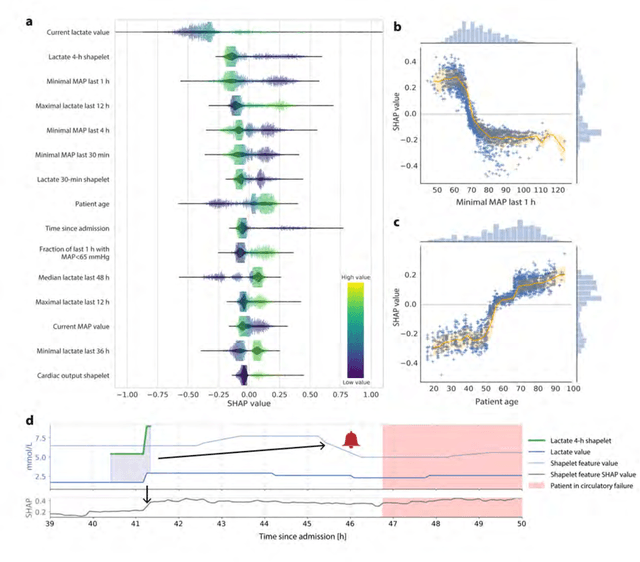
Abstract:Intensive care clinicians are presented with large quantities of patient information and measurements from a multitude of monitoring systems. The limited ability of humans to process such complex information hinders physicians to readily recognize and act on early signs of patient deterioration. We used machine learning to develop an early warning system for circulatory failure based on a high-resolution ICU database with 240 patient years of data. This automatic system predicts 90.0% of circulatory failure events (prevalence 3.1%), with 81.8% identified more than two hours in advance, resulting in an area under the receiver operating characteristic curve of 94.0% and area under the precision-recall curve of 63.0%. The model was externally validated in a large independent patient cohort.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge