Manman Fei
REHRSeg: Unleashing the Power of Self-Supervised Super-Resolution for Resource-Efficient 3D MRI Segmentation
Oct 14, 2024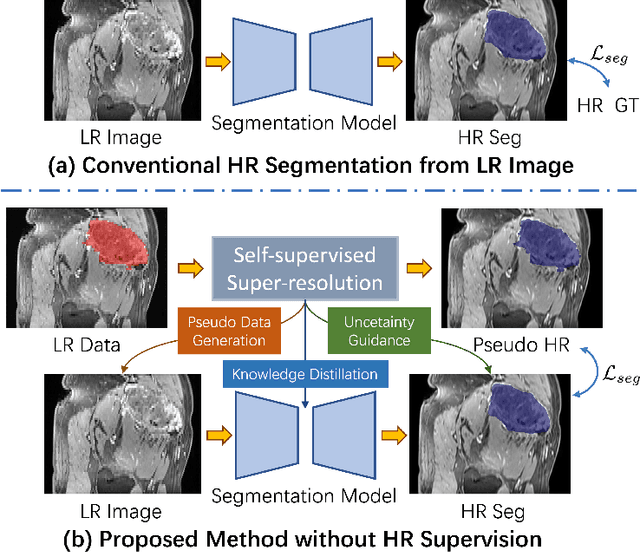
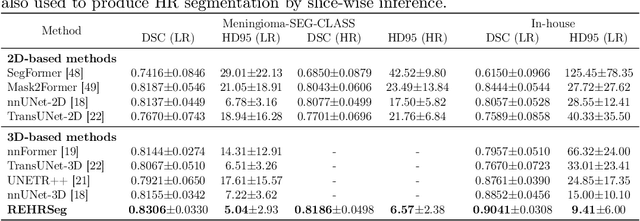
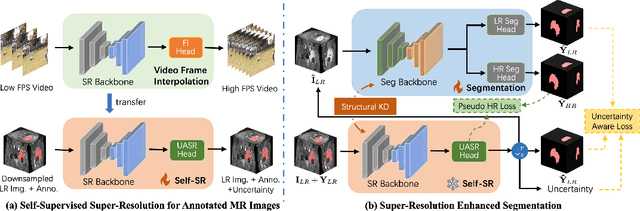
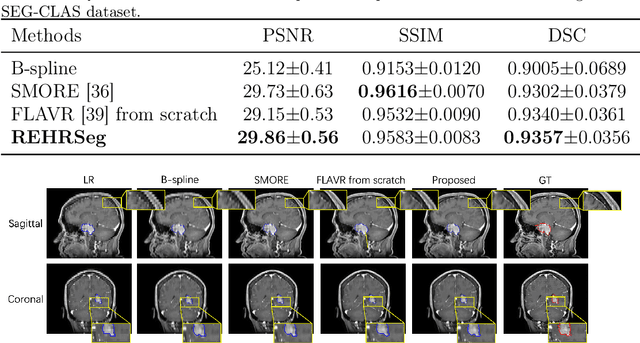
Abstract:High-resolution (HR) 3D magnetic resonance imaging (MRI) can provide detailed anatomical structural information, enabling precise segmentation of regions of interest for various medical image analysis tasks. Due to the high demands of acquisition device, collection of HR images with their annotations is always impractical in clinical scenarios. Consequently, segmentation results based on low-resolution (LR) images with large slice thickness are often unsatisfactory for subsequent tasks. In this paper, we propose a novel Resource-Efficient High-Resolution Segmentation framework (REHRSeg) to address the above-mentioned challenges in real-world applications, which can achieve HR segmentation while only employing the LR images as input. REHRSeg is designed to leverage self-supervised super-resolution (self-SR) to provide pseudo supervision, therefore the relatively easier-to-acquire LR annotated images generated by 2D scanning protocols can be directly used for model training. The main contribution to ensure the effectiveness in self-SR for enhancing segmentation is three-fold: (1) We mitigate the data scarcity problem in the medical field by using pseudo-data for training the segmentation model. (2) We design an uncertainty-aware super-resolution (UASR) head in self-SR to raise the awareness of segmentation uncertainty as commonly appeared on the ROI boundaries. (3) We align the spatial features for self-SR and segmentation through structural knowledge distillation to enable a better capture of region correlations. Experimental results demonstrate that REHRSeg achieves high-quality HR segmentation without intensive supervision, while also significantly improving the baseline performance for LR segmentation.
Gaze-DETR: Using Expert Gaze to Reduce False Positives in Vulvovaginal Candidiasis Screening
May 15, 2024Abstract:Accurate detection of vulvovaginal candidiasis is critical for women's health, yet its sparse distribution and visually ambiguous characteristics pose significant challenges for accurate identification by pathologists and neural networks alike. Our eye-tracking data reveals that areas garnering sustained attention - yet not marked by experts after deliberation - are often aligned with false positives of neural networks. Leveraging this finding, we introduce Gaze-DETR, a pioneering method that integrates gaze data to enhance neural network precision by diminishing false positives. Gaze-DETR incorporates a universal gaze-guided warm-up protocol applicable across various detection methods and a gaze-guided rectification strategy specifically designed for DETR-based models. Our comprehensive tests confirm that Gaze-DETR surpasses existing leading methods, showcasing remarkable improvements in detection accuracy and generalizability.
Two-stage Cytopathological Image Synthesis for Augmenting Cervical Abnormality Screening
Feb 25, 2024Abstract:Automatic thin-prep cytologic test (TCT) screening can assist pathologists in finding cervical abnormality towards accurate and efficient cervical cancer diagnosis. Current automatic TCT screening systems mostly involve abnormal cervical cell detection, which generally requires large-scale and diverse training data with high-quality annotations to achieve promising performance. Pathological image synthesis is naturally raised to minimize the efforts in data collection and annotation. However, it is challenging to generate realistic large-size cytopathological images while simultaneously synthesizing visually plausible appearances for small-size abnormal cervical cells. In this paper, we propose a two-stage image synthesis framework to create synthetic data for augmenting cervical abnormality screening. In the first Global Image Generation stage, a Normal Image Generator is designed to generate cytopathological images full of normal cervical cells. In the second Local Cell Editing stage, normal cells are randomly selected from the generated images and then are converted to different types of abnormal cells using the proposed Abnormal Cell Synthesizer. Both Normal Image Generator and Abnormal Cell Synthesizer are built upon Stable Diffusion, a pre-trained foundation model for image synthesis, via parameter-efficient fine-tuning methods for customizing cytopathological image contents and extending spatial layout controllability, respectively. Our experiments demonstrate the synthetic image quality, diversity, and controllability of the proposed synthesis framework, and validate its data augmentation effectiveness in enhancing the performance of abnormal cervical cell detection.
Uni-COAL: A Unified Framework for Cross-Modality Synthesis and Super-Resolution of MR Images
Nov 14, 2023



Abstract:Cross-modality synthesis (CMS), super-resolution (SR), and their combination (CMSR) have been extensively studied for magnetic resonance imaging (MRI). Their primary goals are to enhance the imaging quality by synthesizing the desired modality and reducing the slice thickness. Despite the promising synthetic results, these techniques are often tailored to specific tasks, thereby limiting their adaptability to complex clinical scenarios. Therefore, it is crucial to build a unified network that can handle various image synthesis tasks with arbitrary requirements of modality and resolution settings, so that the resources for training and deploying the models can be greatly reduced. However, none of the previous works is capable of performing CMS, SR, and CMSR using a unified network. Moreover, these MRI reconstruction methods often treat alias frequencies improperly, resulting in suboptimal detail restoration. In this paper, we propose a Unified Co-Modulated Alias-free framework (Uni-COAL) to accomplish the aforementioned tasks with a single network. The co-modulation design of the image-conditioned and stochastic attribute representations ensures the consistency between CMS and SR, while simultaneously accommodating arbitrary combinations of input/output modalities and thickness. The generator of Uni-COAL is also designed to be alias-free based on the Shannon-Nyquist signal processing framework, ensuring effective suppression of alias frequencies. Additionally, we leverage the semantic prior of Segment Anything Model (SAM) to guide Uni-COAL, ensuring a more authentic preservation of anatomical structures during synthesis. Experiments on three datasets demonstrate that Uni-COAL outperforms the alternatives in CMS, SR, and CMSR tasks for MR images, which highlights its generalizability to wide-range applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge