Mériam Koob
Department of Radiology, Lausanne University Hospital
Advances in Automated Fetal Brain MRI Segmentation and Biometry: Insights from the FeTA 2024 Challenge
May 05, 2025



Abstract:Accurate fetal brain tissue segmentation and biometric analysis are essential for studying brain development in utero. The FeTA Challenge 2024 advanced automated fetal brain MRI analysis by introducing biometry prediction as a new task alongside tissue segmentation. For the first time, our diverse multi-centric test set included data from a new low-field (0.55T) MRI dataset. Evaluation metrics were also expanded to include the topology-specific Euler characteristic difference (ED). Sixteen teams submitted segmentation methods, most of which performed consistently across both high- and low-field scans. However, longitudinal trends indicate that segmentation accuracy may be reaching a plateau, with results now approaching inter-rater variability. The ED metric uncovered topological differences that were missed by conventional metrics, while the low-field dataset achieved the highest segmentation scores, highlighting the potential of affordable imaging systems when paired with high-quality reconstruction. Seven teams participated in the biometry task, but most methods failed to outperform a simple baseline that predicted measurements based solely on gestational age, underscoring the challenge of extracting reliable biometric estimates from image data alone. Domain shift analysis identified image quality as the most significant factor affecting model generalization, with super-resolution pipelines also playing a substantial role. Other factors, such as gestational age, pathology, and acquisition site, had smaller, though still measurable, effects. Overall, FeTA 2024 offers a comprehensive benchmark for multi-class segmentation and biometry estimation in fetal brain MRI, underscoring the need for data-centric approaches, improved topological evaluation, and greater dataset diversity to enable clinically robust and generalizable AI tools.
Automatic quality control in multi-centric fetal brain MRI super-resolution reconstruction
Mar 13, 2025Abstract:Quality control (QC) has long been considered essential to guarantee the reliability of neuroimaging studies. It is particularly important for fetal brain MRI, where acquisitions and image processing techniques are less standardized than in adult imaging. In this work, we focus on automated quality control of super-resolution reconstruction (SRR) volumes of fetal brain MRI, an important processing step where multiple stacks of thick 2D slices are registered together and combined to build a single, isotropic and artifact-free T2 weighted volume. We propose FetMRQC$_{SR}$, a machine-learning method that extracts more than 100 image quality metrics to predict image quality scores using a random forest model. This approach is well suited to a problem that is high dimensional, with highly heterogeneous data and small datasets. We validate FetMRQC$_{SR}$ in an out-of-domain (OOD) setting and report high performance (ROC AUC = 0.89), even when faced with data from an unknown site or SRR method. We also investigate failure cases and show that they occur in $45\%$ of the images due to ambiguous configurations for which the rating from the expert is arguable. These results are encouraging and illustrate how a non deep learning-based method like FetMRQC$_{SR}$ is well suited to this multifaceted problem. Our tool, along with all the code used to generate, train and evaluate the model will be released upon acceptance of the paper.
FetMRQC: an open-source machine learning framework for multi-centric fetal brain MRI quality control
Nov 08, 2023Abstract:Fetal brain MRI is becoming an increasingly relevant complement to neurosonography for perinatal diagnosis, allowing fundamental insights into fetal brain development throughout gestation. However, uncontrolled fetal motion and heterogeneity in acquisition protocols lead to data of variable quality, potentially biasing the outcome of subsequent studies. We present FetMRQC, an open-source machine-learning framework for automated image quality assessment and quality control that is robust to domain shifts induced by the heterogeneity of clinical data. FetMRQC extracts an ensemble of quality metrics from unprocessed anatomical MRI and combines them to predict experts' ratings using random forests. We validate our framework on a pioneeringly large and diverse dataset of more than 1600 manually rated fetal brain T2-weighted images from four clinical centers and 13 different scanners. Our study shows that FetMRQC's predictions generalize well to unseen data while being interpretable. FetMRQC is a step towards more robust fetal brain neuroimaging, which has the potential to shed new insights on the developing human brain.
Multi-dimensional topological loss for cortical plate segmentation in fetal brain MRI
Aug 16, 2022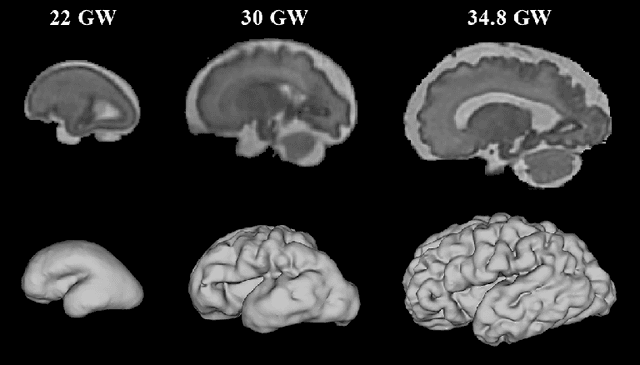
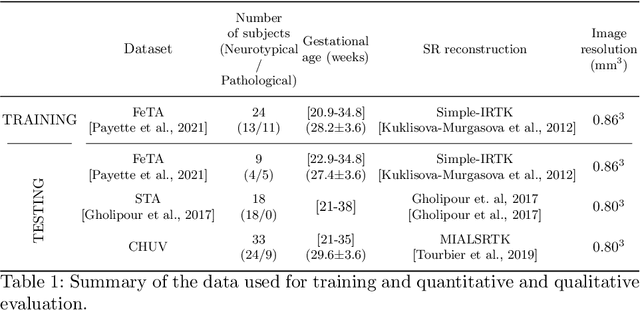
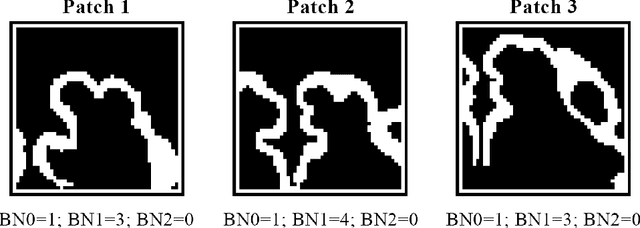
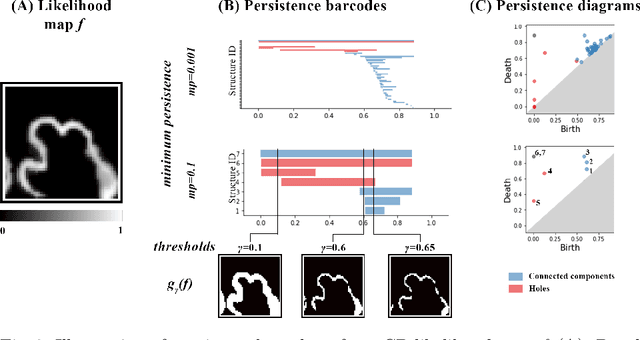
Abstract:The fetal cortical plate (CP) undergoes drastic morphological changes during the in utero development. Therefore, CP growth and folding patterns are key indicator in the assessment of the brain development and maturation. Magnetic resonance imaging (MRI) offers specific insights for the analysis of quantitative imaging biomarkers. Nonetheless, accurate and, more importantly, topologically correct MR image segmentation remains the key baseline to such analysis. In this study, we propose a deep learning segmentation framework for automatic and morphologically consistent segmentation of the CP in fetal brain MRI. Our contribution is two fold. First, we generalized a multi-dimensional topological loss function in order to enhance the topological accuracy. Second, we introduced hole ratio, a new topology-based validation measure that quantifies the size of the topological defects taking into account the size of the structure of interest. Using two publicly available datasets, we quantitatively evaluated our proposed method based on three complementary metrics which are overlap-, distance- and topology-based on 27 fetal brains. Our results evidence that our topology-integrative framework outperforms state-of-the-art training loss functions on super-resolution reconstructed clinical MRI, not only in shape correctness but also in the classical evaluation metrics. Furthermore, results on additional 31 out-of-domain SR reconstructions from clinical acquisitions were qualitatively assessed by three experts. The experts' consensus ranked our TopoCP method as the best segmentation in 100\% of the cases with a high inter-expert agreement. Overall, both quantitative and qualitative results, on a wide range of gestational ages and number of cases, support the generalizability and added value of our topology-guided framework for fetal CP segmentation.
FaBiAN: A Fetal Brain magnetic resonance Acquisition Numerical phantom
Sep 06, 2021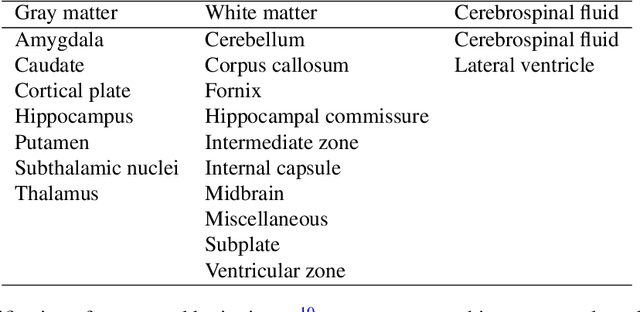
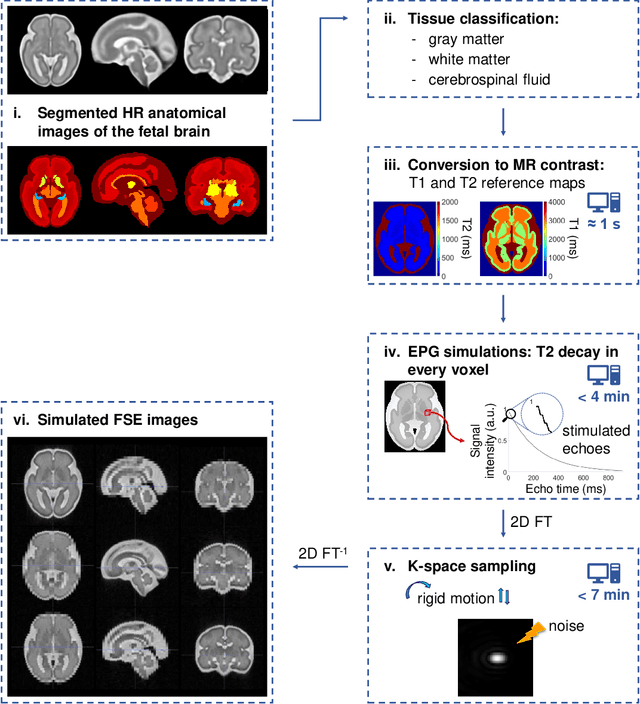
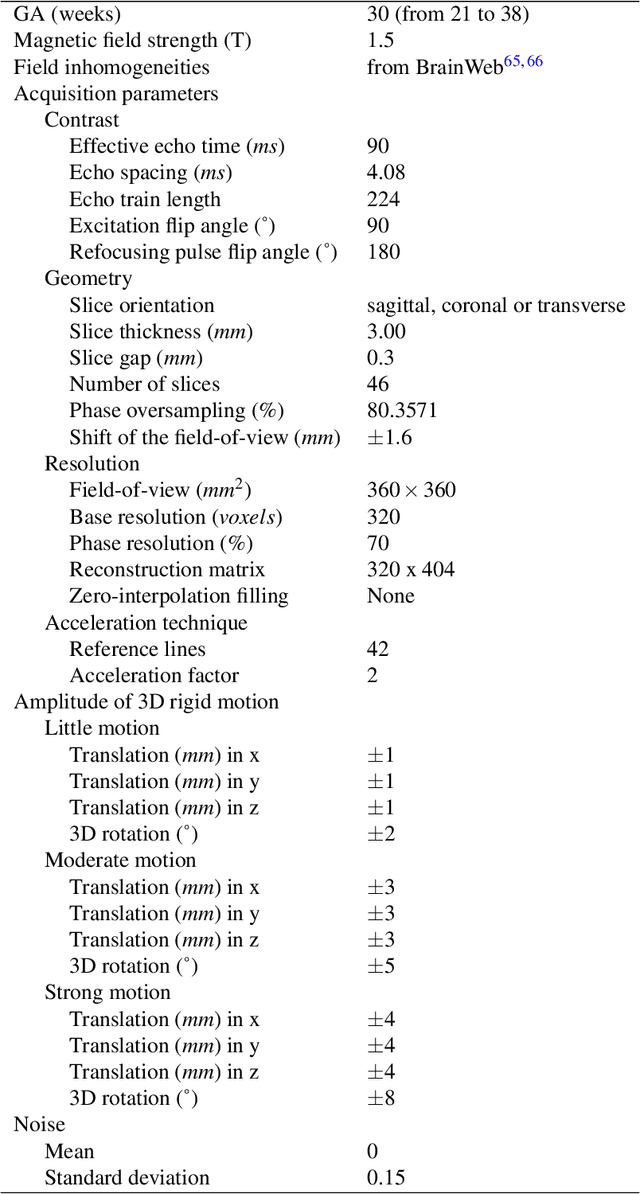
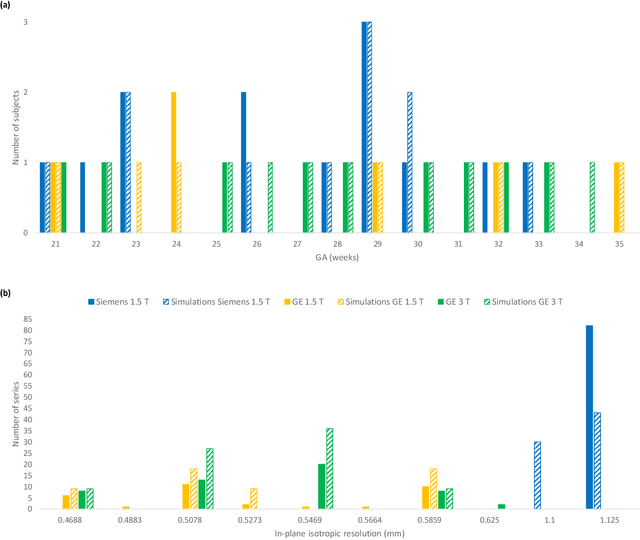
Abstract:Accurate characterization of in utero human brain maturation is critical as it involves complex and interconnected structural and functional processes that may influence health later in life. Magnetic resonance imaging is a powerful tool to investigate equivocal neurological patterns during fetal development. However, the number of acquisitions of satisfactory quality available in this cohort of sensitive subjects remains scarce, thus hindering the validation of advanced image processing techniques. Numerical phantoms can mitigate these limitations by providing a controlled environment with a known ground truth. In this work, we present FaBiAN, an open-source Fetal Brain magnetic resonance Acquisition Numerical phantom that simulates clinical T2-weighted fast spin echo sequences of the fetal brain. This unique tool is based on a general, flexible and realistic setup that includes stochastic fetal movements, thus providing images of the fetal brain throughout maturation comparable to clinical acquisitions. We demonstrate its value to evaluate the robustness and optimize the accuracy of an algorithm for super-resolution fetal brain magnetic resonance imaging from simulated motion-corrupted 2D low-resolution series as compared to a synthetic high-resolution reference volume. We also show that the images generated can complement clinical datasets to support data-intensive deep learning methods for fetal brain tissue segmentation.
Segmentation of the cortical plate in fetal brain MRI with a topological loss
Oct 23, 2020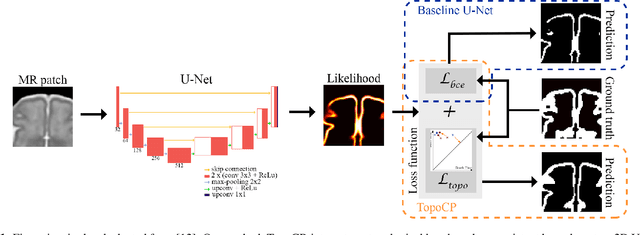

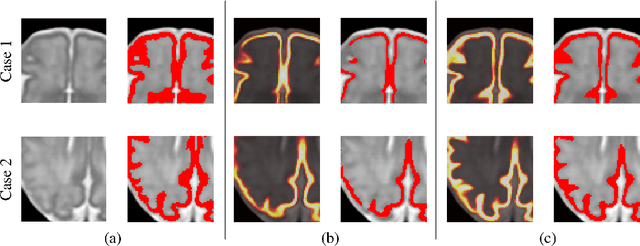

Abstract:The fetal cortical plate undergoes drastic morphological changes throughout early in utero development that can be observed using magnetic resonance (MR) imaging. An accurate MR image segmentation, and more importantly a topologically correct delineation of the cortical gray matter, is a key baseline to perform further quantitative analysis of brain development. In this paper, we propose for the first time the integration of a topological constraint, as an additional loss function, to enhance the morphological consistency of a deep learning-based segmentation of the fetal cortical plate. We quantitatively evaluate our method on 18 fetal brain atlases ranging from 21 to 38 weeks of gestation, showing the significant benefits of our method through all gestational ages as compared to a baseline method. Furthermore, qualitative evaluation by three different experts on 130 randomly selected slices from 26 clinical MRIs evidences the out-performance of our method independently of the MR reconstruction quality.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge