Chirine Atat
Transfer learning with weak labels from radiology reports: application to glioma change detection
Oct 18, 2022
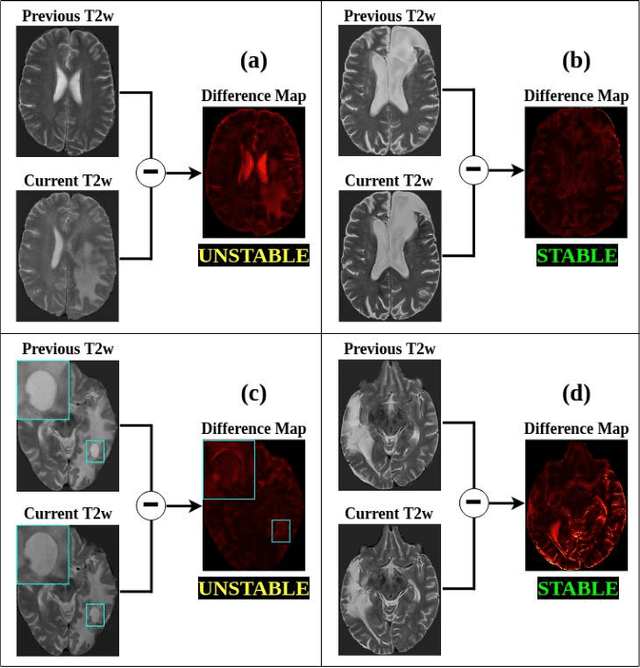


Abstract:Creating large annotated datasets represents a major bottleneck for the development of deep learning models in radiology. To overcome this, we propose a combined use of weak labels (imprecise, but fast-to-create annotations) and Transfer Learning (TL). Specifically, we explore inductive TL, where source and target domains are identical, but tasks are different due to a label shift: our target labels are created manually by three radiologists, whereas the source weak labels are generated automatically from textual radiology reports. We frame knowledge transfer as hyperparameter optimization, thus avoiding heuristic choices that are frequent in related works. We investigate the relationship between model size and TL, comparing a low-capacity VGG with a higher-capacity SEResNeXt. The task that we address is change detection in follow-up glioma imaging: we extracted 1693 T2-weighted magnetic resonance imaging difference maps from 183 patients, and classified them into stable or unstable according to tumor evolution. Weak labeling allowed us to increase dataset size more than 3-fold, and improve VGG classification results from 75% to 82% Area Under the ROC Curve (AUC) (p=0.04). Mixed training from scratch led to higher performance than fine-tuning or feature extraction. To assess generalizability, we also ran inference on an open dataset (BraTS-2015: 15 patients, 51 difference maps), reaching up to 76% AUC. Overall, results suggest that medical imaging problems may benefit from smaller models and different TL strategies with respect to computer vision problems, and that report-generated weak labels are effective in improving model performances. Code, in-house dataset and BraTS labels are released.
Segmentation of the cortical plate in fetal brain MRI with a topological loss
Oct 23, 2020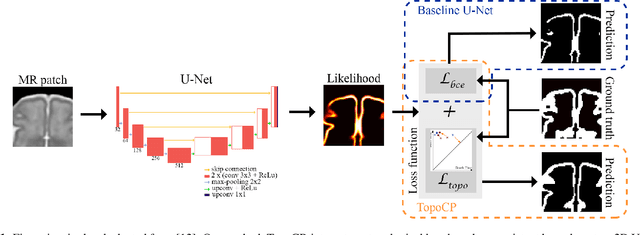

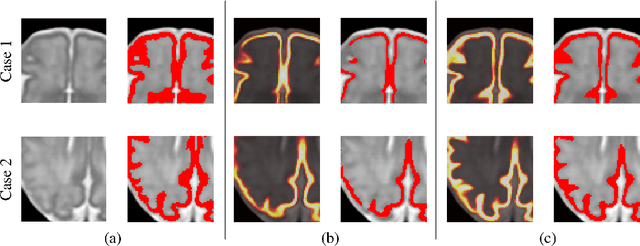

Abstract:The fetal cortical plate undergoes drastic morphological changes throughout early in utero development that can be observed using magnetic resonance (MR) imaging. An accurate MR image segmentation, and more importantly a topologically correct delineation of the cortical gray matter, is a key baseline to perform further quantitative analysis of brain development. In this paper, we propose for the first time the integration of a topological constraint, as an additional loss function, to enhance the morphological consistency of a deep learning-based segmentation of the fetal cortical plate. We quantitatively evaluate our method on 18 fetal brain atlases ranging from 21 to 38 weeks of gestation, showing the significant benefits of our method through all gestational ages as compared to a baseline method. Furthermore, qualitative evaluation by three different experts on 130 randomly selected slices from 26 clinical MRIs evidences the out-performance of our method independently of the MR reconstruction quality.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge