Loren Laine
Integrating Expert Judgment and Algorithmic Decision Making: An Indistinguishability Framework
Oct 11, 2024


Abstract:We introduce a novel framework for human-AI collaboration in prediction and decision tasks. Our approach leverages human judgment to distinguish inputs which are algorithmically indistinguishable, or "look the same" to any feasible predictive algorithm. We argue that this framing clarifies the problem of human-AI collaboration in prediction and decision tasks, as experts often form judgments by drawing on information which is not encoded in an algorithm's training data. Algorithmic indistinguishability yields a natural test for assessing whether experts incorporate this kind of "side information", and further provides a simple but principled method for selectively incorporating human feedback into algorithmic predictions. We show that this method provably improves the performance of any feasible algorithmic predictor and precisely quantify this improvement. We demonstrate the utility of our framework in a case study of emergency room triage decisions, where we find that although algorithmic risk scores are highly competitive with physicians, there is strong evidence that physician judgments provide signal which could not be replicated by any predictive algorithm. This insight yields a range of natural decision rules which leverage the complementary strengths of human experts and predictive algorithms.
Assessing the Usability of GutGPT: A Simulation Study of an AI Clinical Decision Support System for Gastrointestinal Bleeding Risk
Dec 06, 2023Abstract:Applications of large language models (LLMs) like ChatGPT have potential to enhance clinical decision support through conversational interfaces. However, challenges of human-algorithmic interaction and clinician trust are poorly understood. GutGPT, a LLM for gastrointestinal (GI) bleeding risk prediction and management guidance, was deployed in clinical simulation scenarios alongside the electronic health record (EHR) with emergency medicine physicians, internal medicine physicians, and medical students to evaluate its effect on physician acceptance and trust in AI clinical decision support systems (AI-CDSS). GutGPT provides risk predictions from a validated machine learning model and evidence-based answers by querying extracted clinical guidelines. Participants were randomized to GutGPT and an interactive dashboard, or the interactive dashboard and a search engine. Surveys and educational assessments taken before and after measured technology acceptance and content mastery. Preliminary results showed mixed effects on acceptance after using GutGPT compared to the dashboard or search engine but appeared to improve content mastery based on simulation performance. Overall, this study demonstrates LLMs like GutGPT could enhance effective AI-CDSS if implemented optimally and paired with interactive interfaces.
Auditing for Human Expertise
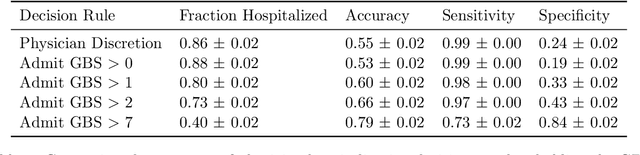
Jun 02, 2023Abstract:High-stakes prediction tasks (e.g., patient diagnosis) are often handled by trained human experts. A common source of concern about automation in these settings is that experts may exercise intuition that is difficult to model and/or have access to information (e.g., conversations with a patient) that is simply unavailable to a would-be algorithm. This raises a natural question whether human experts add value which could not be captured by an algorithmic predictor. We develop a statistical framework under which we can pose this question as a natural hypothesis test. Indeed, as our framework highlights, detecting human expertise is more subtle than simply comparing the accuracy of expert predictions to those made by a particular learning algorithm. Instead, we propose a simple procedure which tests whether expert predictions are statistically independent from the outcomes of interest after conditioning on the available inputs (`features'). A rejection of our test thus suggests that human experts may add value to any algorithm trained on the available data, and has direct implications for whether human-AI `complementarity' is achievable in a given prediction task. We highlight the utility of our procedure using admissions data collected from the emergency department of a large academic hospital system, where we show that physicians' admit/discharge decisions for patients with acute gastrointestinal bleeding (AGIB) appear to be incorporating information not captured in a standard algorithmic screening tool. This is despite the fact that the screening tool is arguably more accurate than physicians' discretionary decisions, highlighting that -- even absent normative concerns about accountability or interpretability -- accuracy is insufficient to justify algorithmic automation.
MURAL: An Unsupervised Random Forest-Based Embedding for Electronic Health Record Data
Nov 19, 2021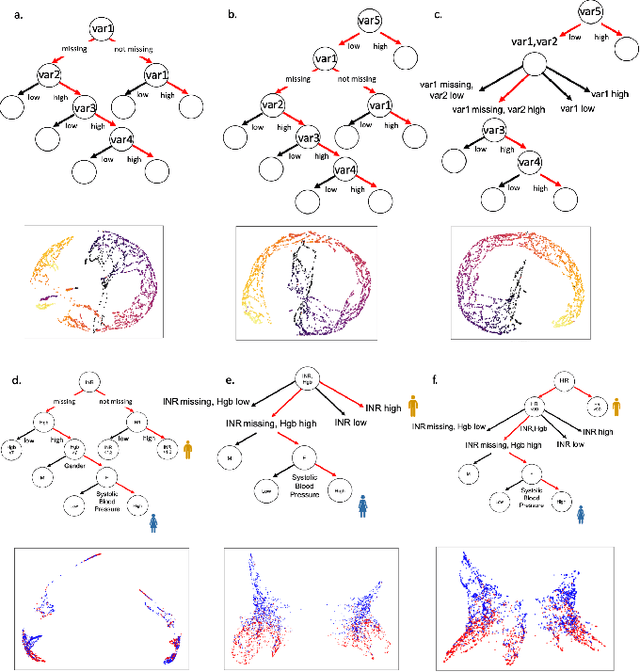
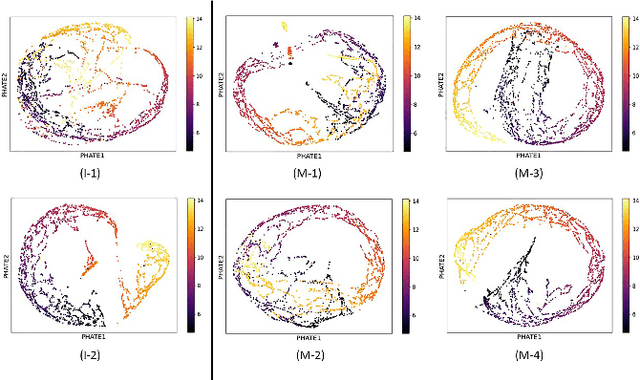
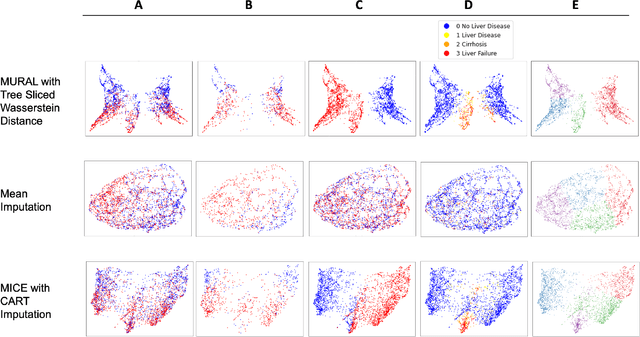

Abstract:A major challenge in embedding or visualizing clinical patient data is the heterogeneity of variable types including continuous lab values, categorical diagnostic codes, as well as missing or incomplete data. In particular, in EHR data, some variables are {\em missing not at random (MNAR)} but deliberately not collected and thus are a source of information. For example, lab tests may be deemed necessary for some patients on the basis of suspected diagnosis, but not for others. Here we present the MURAL forest -- an unsupervised random forest for representing data with disparate variable types (e.g., categorical, continuous, MNAR). MURAL forests consist of a set of decision trees where node-splitting variables are chosen at random, such that the marginal entropy of all other variables is minimized by the split. This allows us to also split on MNAR variables and discrete variables in a way that is consistent with the continuous variables. The end goal is to learn the MURAL embedding of patients using average tree distances between those patients. These distances can be fed to nonlinear dimensionality reduction method like PHATE to derive visualizable embeddings. While such methods are ubiquitous in continuous-valued datasets (like single cell RNA-sequencing) they have not been used extensively in mixed variable data. We showcase the use of our method on one artificial and two clinical datasets. We show that using our approach, we can visualize and classify data more accurately than competing approaches. Finally, we show that MURAL can also be used to compare cohorts of patients via the recently proposed tree-sliced Wasserstein distances.
Making Logic Learnable With Neural Networks
Feb 18, 2020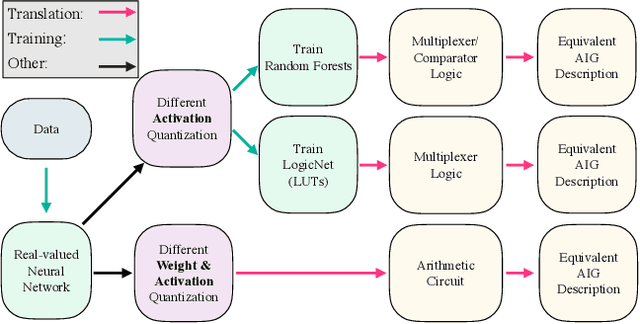
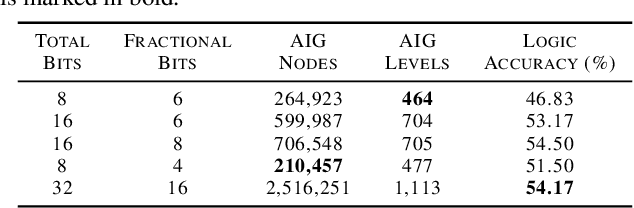
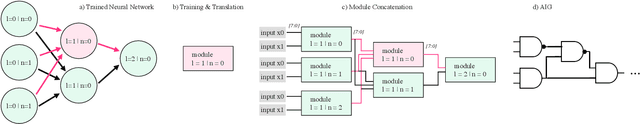
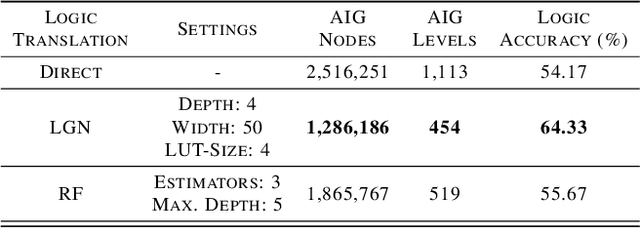
Abstract:While neural networks are good at learning unspecified functions from training samples, they cannot be directly implemented in hardware and are often not interpretable or formally verifiable. On the other hand, logic circuits are implementable, verifiable, and interpretable but are not able to learn from training data in a generalizable way. We propose a novel logic learning pipeline that combines the advantages of neural networks and logic circuits. Our pipeline first trains a neural network on a classification task, and then translates this, first to random forests or look-up tables, and then to AND-Inverter logic. We show that our pipeline maintains greater accuracy than naive translations to logic, and minimizes the logic such that it is more interpretable and has decreased hardware cost. We show the utility of our pipeline on a network that is trained on biomedical data from patients presenting with gastrointestinal bleeding with the prediction task of determining if patients need immediate hospital-based intervention. This approach could be applied to patient care to provide risk stratification and guide clinical decision-making.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge