Jianchun Zhao
Cross-domain Collaborative Learning for Recognizing Multiple Retinal Diseases from Wide-Field Fundus Images
May 14, 2023Abstract:This paper addresses the emerging task of recognizing multiple retinal diseases from wide-field (WF) and ultra-wide-field (UWF) fundus images. For an effective reuse of existing labeled color fundus photo (CFP) data, we propose Cross-domain Collaborative Learning (CdCL). Inspired by the success of fixed-ratio based mixup in unsupervised domain adaptation, we re-purpose this strategy for the current task. Due to the intrinsic disparity between the field-of-view of CFP and WF/UWF images, a scale bias naturally exists in a mixup sample that the anatomic structure from a CFP image will be considerably larger than its WF/UWF counterpart. The CdCL method resolves the issue by Scale-bias Correction, which employs Transformers for producing scale-invariant features. As demonstrated by extensive experiments on multiple datasets covering both WF and UWF images, the proposed method compares favorably against a number of competitive baselines.
Lesion Localization in OCT by Semi-Supervised Object Detection
Apr 24, 2022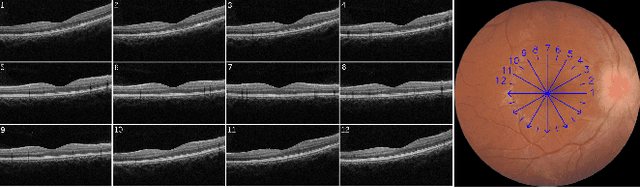
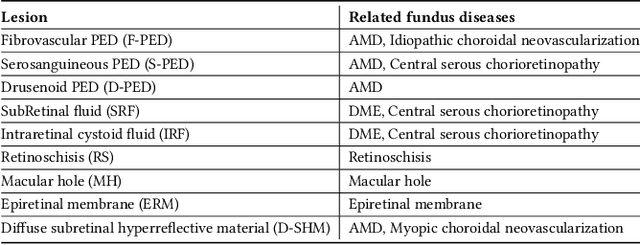
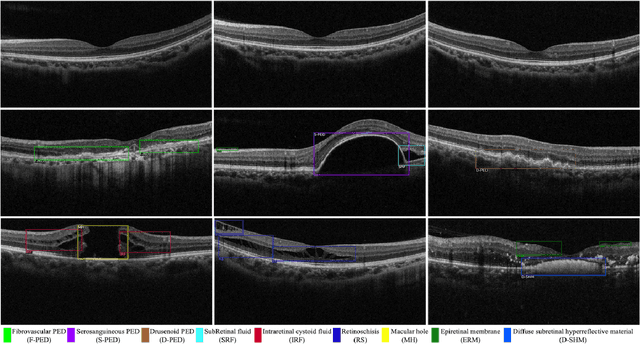
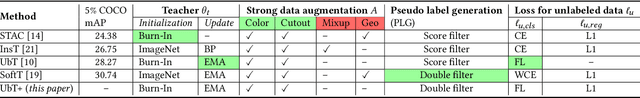
Abstract:Over 300 million people worldwide are affected by various retinal diseases. By noninvasive Optical Coherence Tomography (OCT) scans, a number of abnormal structural changes in the retina, namely retinal lesions, can be identified. Automated lesion localization in OCT is thus important for detecting retinal diseases at their early stage. To conquer the lack of manual annotation for deep supervised learning, this paper presents a first study on utilizing semi-supervised object detection (SSOD) for lesion localization in OCT images. To that end, we develop a taxonomy to provide a unified and structured viewpoint of the current SSOD methods, and consequently identify key modules in these methods. To evaluate the influence of these modules in the new task, we build OCT-SS, a new dataset consisting of over 1k expert-labeled OCT B-scan images and over 13k unlabeled B-scans. Extensive experiments on OCT-SS identify Unbiased Teacher (UnT) as the best current SSOD method for lesion localization. Moreover, we improve over this strong baseline, with mAP increased from 49.34 to 50.86.
Multi-Modal Multi-Instance Learning for Retinal Disease Recognition
Sep 25, 2021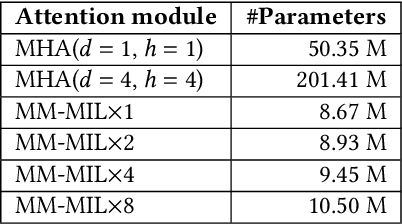
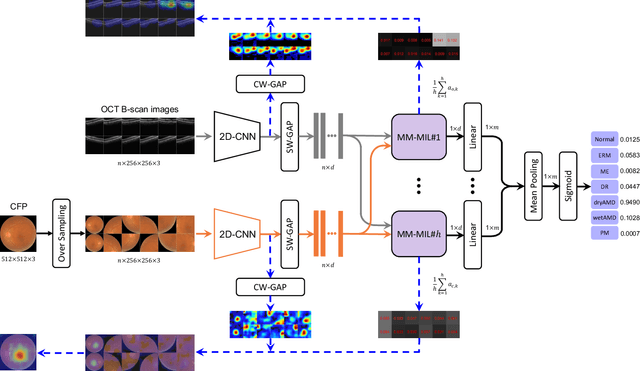
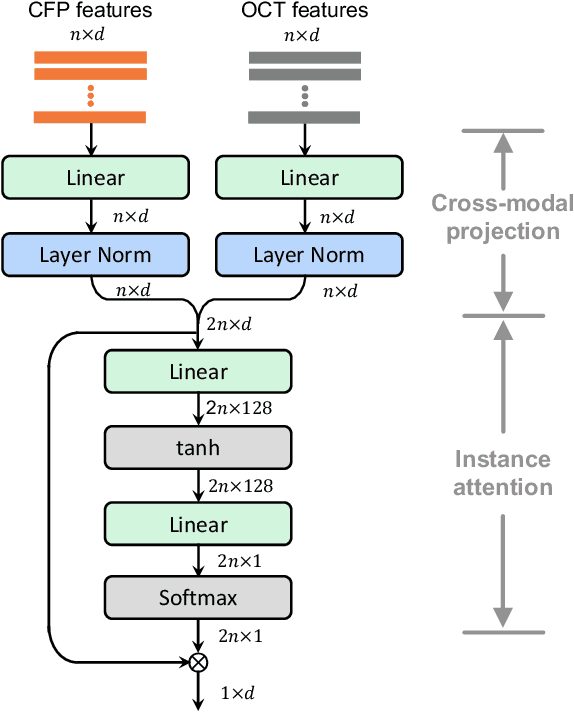
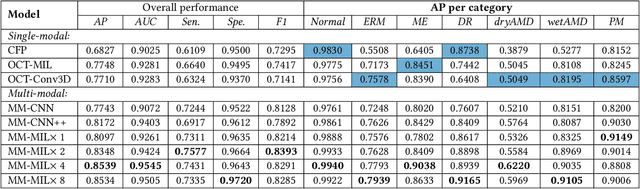
Abstract:This paper attacks an emerging challenge of multi-modal retinal disease recognition. Given a multi-modal case consisting of a color fundus photo (CFP) and an array of OCT B-scan images acquired during an eye examination, we aim to build a deep neural network that recognizes multiple vision-threatening diseases for the given case. As the diagnostic efficacy of CFP and OCT is disease-dependent, the network's ability of being both selective and interpretable is important. Moreover, as both data acquisition and manual labeling are extremely expensive in the medical domain, the network has to be relatively lightweight for learning from a limited set of labeled multi-modal samples. Prior art on retinal disease recognition focuses either on a single disease or on a single modality, leaving multi-modal fusion largely underexplored. We propose in this paper Multi-Modal Multi-Instance Learning (MM-MIL) for selectively fusing CFP and OCT modalities. Its lightweight architecture (as compared to current multi-head attention modules) makes it suited for learning from relatively small-sized datasets. For an effective use of MM-MIL, we propose to generate a pseudo sequence of CFPs by over sampling a given CFP. The benefits of this tactic include well balancing instances across modalities, increasing the resolution of the CFP input, and finding out regions of the CFP most relevant with respect to the final diagnosis. Extensive experiments on a real-world dataset consisting of 1,206 multi-modal cases from 1,193 eyes of 836 subjects demonstrate the viability of the proposed model.
Learning Two-Stream CNN for Multi-Modal Age-related Macular Degeneration Categorization
Dec 03, 2020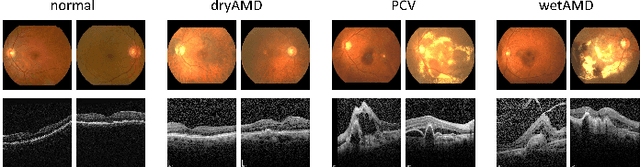
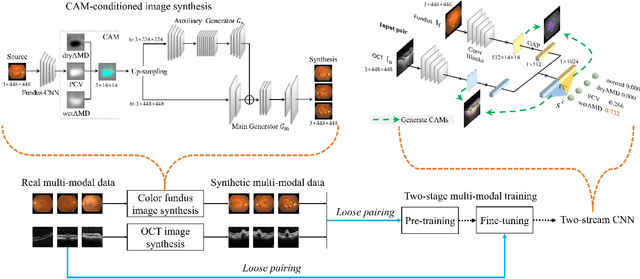
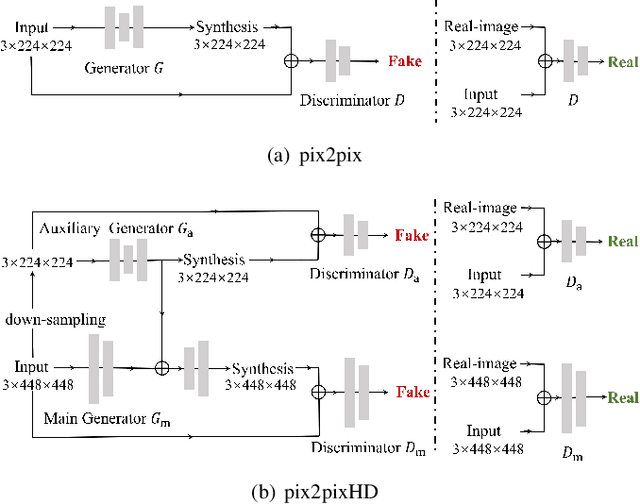
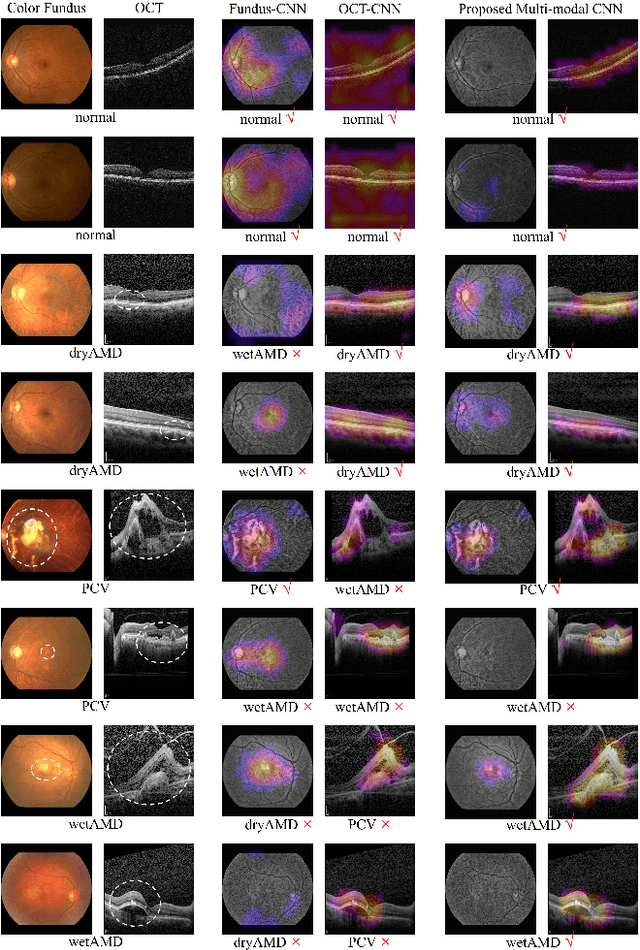
Abstract:This paper tackles automated categorization of Age-related Macular Degeneration (AMD), a common macular disease among people over 50. Previous research efforts mainly focus on AMD categorization with a single-modal input, let it be a color fundus image or an OCT image. By contrast, we consider AMD categorization given a multi-modal input, a direction that is clinically meaningful yet mostly unexplored. Contrary to the prior art that takes a traditional approach of feature extraction plus classifier training that cannot be jointly optimized, we opt for end-to-end multi-modal Convolutional Neural Networks (MM-CNN). Our MM-CNN is instantiated by a two-stream CNN, with spatially-invariant fusion to combine information from the fundus and OCT streams. In order to visually interpret the contribution of the individual modalities to the final prediction, we extend the class activation mapping (CAM) technique to the multi-modal scenario. For effective training of MM-CNN, we develop two data augmentation methods. One is GAN-based fundus / OCT image synthesis, with our novel use of CAMs as conditional input of a high-resolution image-to-image translation GAN. The other method is Loose Pairing, which pairs a fundus image and an OCT image on the basis of their classes instead of eye identities. Experiments on a clinical dataset consisting of 1,099 color fundus images and 1,290 OCT images acquired from 1,099 distinct eyes verify the effectiveness of the proposed solution for multi-modal AMD categorization.
Two-Stream CNN with Loose Pair Training for Multi-modal AMD Categorization
Jul 28, 2019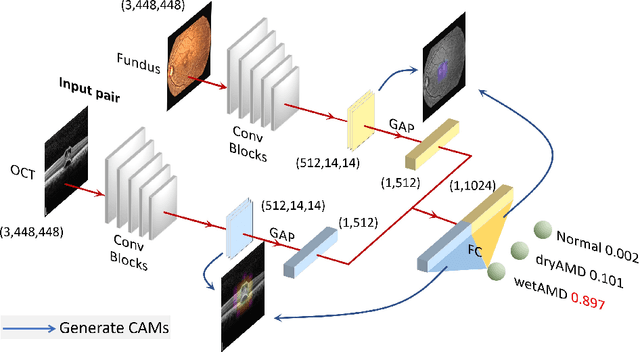
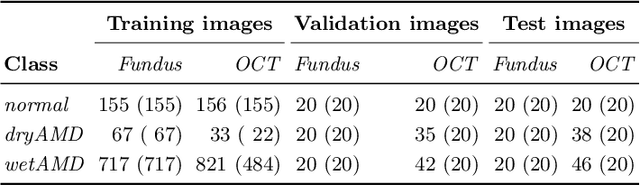

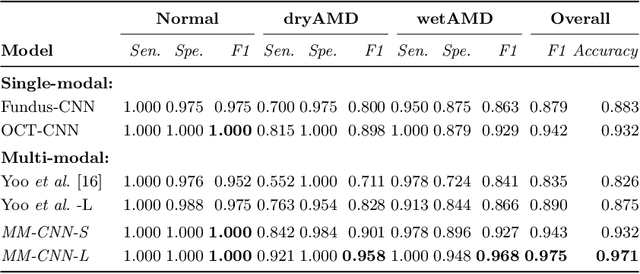
Abstract:This paper studies automated categorization of age-related macular degeneration (AMD) given a multi-modal input, which consists of a color fundus image and an optical coherence tomography (OCT) image from a specific eye. Previous work uses a traditional method, comprised of feature extraction and classifier training that cannot be optimized jointly. By contrast, we propose a two-stream convolutional neural network (CNN) that is end-to-end. The CNN's fusion layer is tailored to the need of fusing information from the fundus and OCT streams. For generating more multi-modal training instances, we introduce Loose Pair training, where a fundus image and an OCT image are paired based on class labels rather than eyes. Moreover, for a visual interpretation of how the individual modalities make contributions, we extend the class activation mapping technique to the multi-modal scenario. Experiments on a real-world dataset collected from an outpatient clinic justify the viability of our proposal for multi-modal AMD categorization.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge