Huabin Zhang
CEST-KAN: Kolmogorov-Arnold Networks for CEST MRI Data Analysis
Jun 25, 2024



Abstract:Purpose: This study aims to propose and investigate the feasibility of using Kolmogorov-Arnold Network (KAN) for CEST MRI data analysis (CEST-KAN). Methods: CEST MRI data were acquired from twelve healthy volunteers at 3T. Data from ten subjects were used for training, while the remaining two were reserved for testing. The performance of multi-layer perceptron (MLP) and KAN models with the same network settings were evaluated and compared to the conventional multi-pool Lorentzian fitting (MPLF) method in generating water and multiple CEST contrasts, including amide, relayed nuclear Overhauser effect (rNOE), and magnetization transfer (MT). Results: The water and CEST maps generated by both MLP and KAN were visually comparable to the MPLF results. However, the KAN model demonstrated higher accuracy in extrapolating the CEST fitting metrics, as evidenced by the smaller validation loss during training and smaller absolute error during testing. Voxel-wise correlation analysis showed that all four CEST fitting metrics generated by KAN consistently exhibited higher Pearson coefficients than the MLP results, indicating superior performance. Moreover, the KAN models consistently outperformed the MLP models in varying hidden layer numbers despite longer training time. Conclusion: In this study, we demonstrated for the first time the feasibility of utilizing KAN for CEST MRI data analysis, highlighting its superiority over MLP in this task. The findings suggest that CEST-KAN has the potential to be a robust and reliable post-analysis tool for CEST MRI in clinical settings.
Dual-Channel Reliable Breast Ultrasound Image Classification Based on Explainable Attribution and Uncertainty Quantification
Jan 08, 2024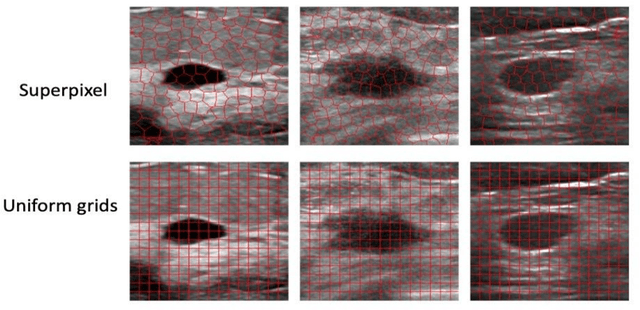
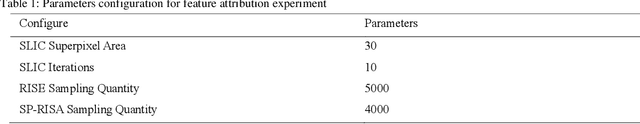
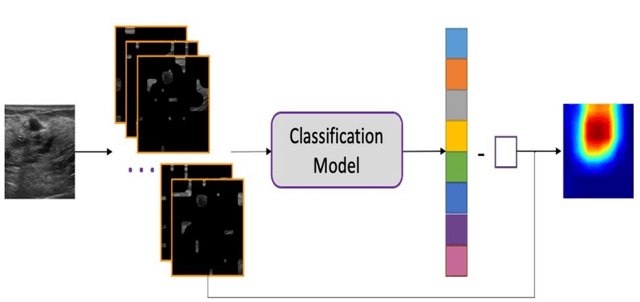

Abstract:This paper focuses on the classification task of breast ultrasound images and researches on the reliability measurement of classification results. We proposed a dual-channel evaluation framework based on the proposed inference reliability and predictive reliability scores. For the inference reliability evaluation, human-aligned and doctor-agreed inference rationales based on the improved feature attribution algorithm SP-RISA are gracefully applied. Uncertainty quantification is used to evaluate the predictive reliability via the Test Time Enhancement. The effectiveness of this reliability evaluation framework has been verified on our breast ultrasound clinical dataset YBUS, and its robustness is verified on the public dataset BUSI. The expected calibration errors on both datasets are significantly lower than traditional evaluation methods, which proves the effectiveness of our proposed reliability measurement.
BSM loss: A superior way in modeling aleatory uncertainty of fine_grained classification
Jun 09, 2022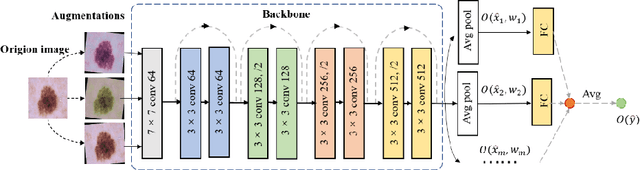
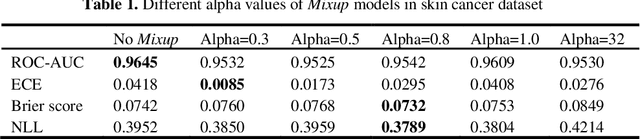
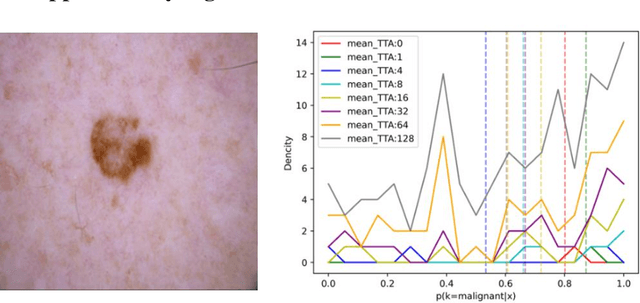
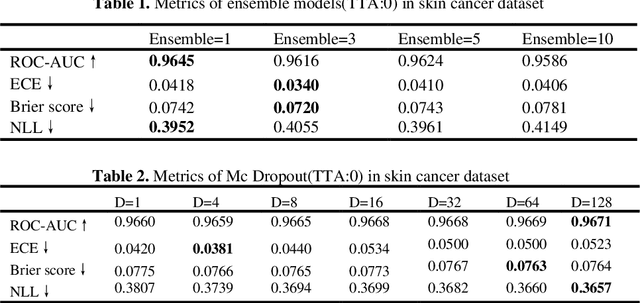
Abstract:Artificial intelligence(AI)-assisted method had received much attention in the risk field such as disease diagnosis. Different from the classification of disease types, it is a fine-grained task to classify the medical images as benign or malignant. However, most research only focuses on improving the diagnostic accuracy and ignores the evaluation of model reliability, which limits its clinical application. For clinical practice, calibration presents major challenges in the low-data regime extremely for over-parametrized models and inherent noises. In particular, we discovered that modeling data-dependent uncertainty is more conducive to confidence calibrations. Compared with test-time augmentation(TTA), we proposed a modified Bootstrapping loss(BS loss) function with Mixup data augmentation strategy that can better calibrate predictive uncertainty and capture data distribution transformation without additional inference time. Our experiments indicated that BS loss with Mixup(BSM) model can halve the Expected Calibration Error(ECE) compared to standard data augmentation, deep ensemble and MC dropout. The correlation between uncertainty and similarity of in-domain data is up to -0.4428 under the BSM model. Additionally, the BSM model is able to perceive the semantic distance of out-of-domain data, demonstrating high potential in real-world clinical practice.
AI assisted method for efficiently generating breast ultrasound screening reports
Jul 28, 2021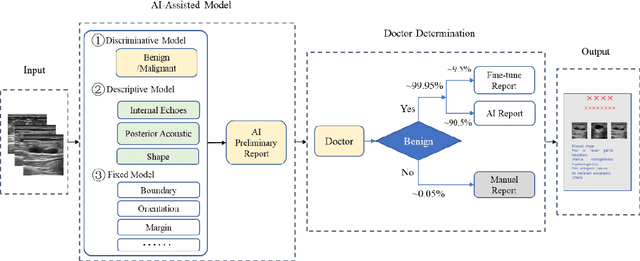

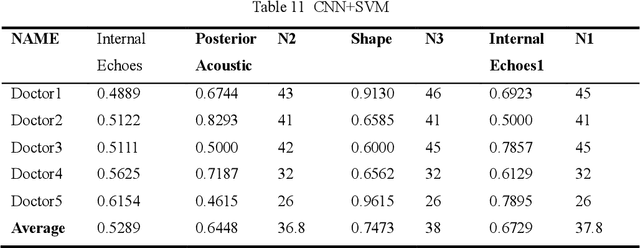
Abstract:Ultrasound is the preferred choice for early screening of dense breast cancer. Clinically, doctors have to manually write the screening report which is time-consuming and laborious, and it is easy to miss and miswrite. Therefore, this paper proposes a method for efficiently generating personalized breast ultrasound screening preliminary reports by AI, especially for benign and normal cases which account for the majority. Doctors then make simple adjustments or corrections to quickly generate final reports. The proposed approach has been tested using a database of 1133 breast tumor instances. Experimental results indicate this pipeline improves doctors' work efficiency by up to 90%, which greatly reduces repetitive work.
More Reliable AI Solution: Breast Ultrasound Diagnosis Using Multi-AI Combination
Jan 07, 2021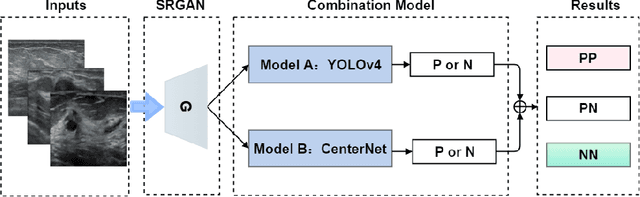
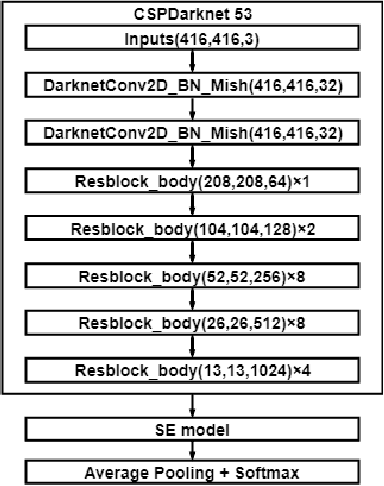
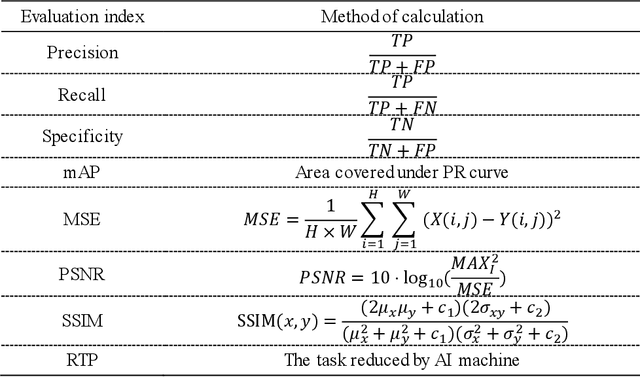
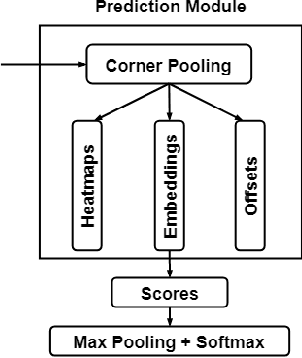
Abstract:Objective: Breast cancer screening is of great significance in contemporary women's health prevention. The existing machines embedded in the AI system do not reach the accuracy that clinicians hope. How to make intelligent systems more reliable is a common problem. Methods: 1) Ultrasound image super-resolution: the SRGAN super-resolution network reduces the unclearness of ultrasound images caused by the device itself and improves the accuracy and generalization of the detection model. 2) In response to the needs of medical images, we have improved the YOLOv4 and the CenterNet models. 3) Multi-AI model: based on the respective advantages of different AI models, we employ two AI models to determine clinical resuls cross validation. And we accept the same results and refuses others. Results: 1) With the help of the super-resolution model, the YOLOv4 model and the CenterNet model both increased the mAP score by 9.6% and 13.8%. 2) Two methods for transforming the target model into a classification model are proposed. And the unified output is in a specified format to facilitate the call of the molti-AI model. 3) In the classification evaluation experiment, concatenated by the YOLOv4 model (sensitivity 57.73%, specificity 90.08%) and the CenterNet model (sensitivity 62.64%, specificity 92.54%), the multi-AI model will refuse to make judgments on 23.55% of the input data. Correspondingly, the performance has been greatly improved to 95.91% for the sensitivity and 96.02% for the specificity. Conclusion: Our work makes the AI model more reliable in medical image diagnosis. Significance: 1) The proposed method makes the target detection model more suitable for diagnosing breast ultrasound images. 2) It provides a new idea for artificial intelligence in medical diagnosis, which can more conveniently introduce target detection models from other fields to serve medical lesion screening.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge