Daniel Bos
Department of Radiology and Nuclear Medicine, Erasmus MC, Rotterdam, The Netherlands, Department of Epidemiology, Erasmus MC, Rotterdam, The Netherlands
MyDigiTwin: A Privacy-Preserving Framework for Personalized Cardiovascular Risk Prediction and Scenario Exploration
Jan 21, 2025



Abstract:Cardiovascular disease (CVD) remains a leading cause of death, and primary prevention through personalized interventions is crucial. This paper introduces MyDigiTwin, a framework that integrates health digital twins with personal health environments to empower patients in exploring personalized health scenarios while ensuring data privacy. MyDigiTwin uses federated learning to train predictive models across distributed datasets without transferring raw data, and a novel data harmonization framework addresses semantic and format inconsistencies in health data. A proof-of-concept demonstrates the feasibility of harmonizing and using cohort data to train privacy-preserving CVD prediction models. This framework offers a scalable solution for proactive, personalized cardiovascular care and sets the stage for future applications in real-world healthcare settings.
Computer-aided diagnosis and prediction in brain disorders
Jun 29, 2022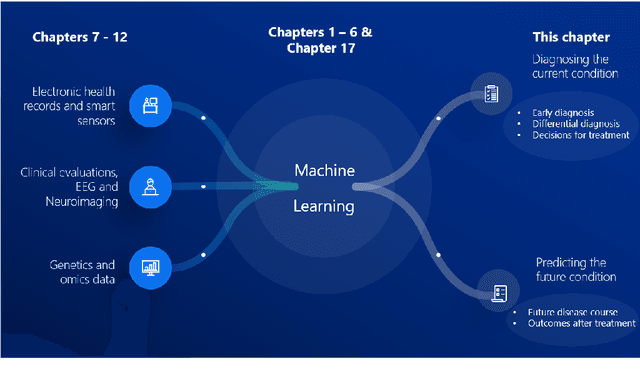
Abstract:Computer-aided methods have shown added value for diagnosing and predicting brain disorders and can thus support decision making in clinical care and treatment planning. This chapter will provide insight into the type of methods, their working, their input data - such as cognitive tests, imaging and genetic data - and the types of output they provide. We will focus on specific use cases for diagnosis, i.e. estimating the current 'condition' of the patient, such as early detection and diagnosis of dementia, differential diagnosis of brain tumours, and decision making in stroke. Regarding prediction, i.e. estimation of the future 'condition' of the patient, we will zoom in on use cases such as predicting the disease course in multiple sclerosis and predicting patient outcomes after treatment in brain cancer. Furthermore, based on these use cases, we will assess the current state-of-the-art methodology and highlight current efforts on benchmarking of these methods and the importance of open science therein. Finally, we assess the current clinical impact of computer-aided methods and discuss the required next steps to increase clinical impact.
A Quantitative Comparison of Epistemic Uncertainty Maps Applied to Multi-Class Segmentation
Sep 22, 2021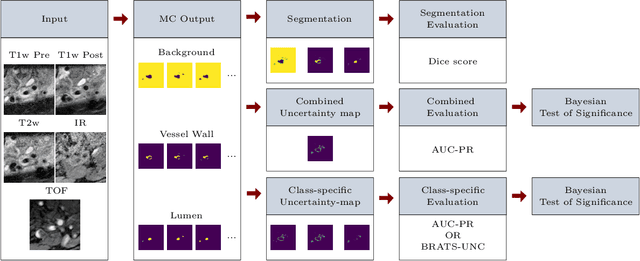
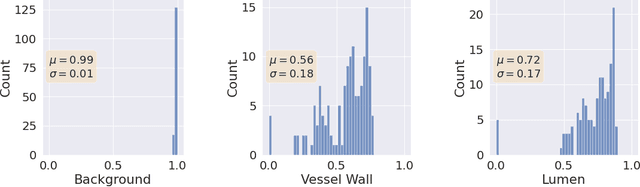
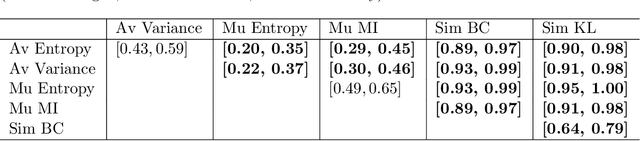
Abstract:Uncertainty assessment has gained rapid interest in medical image analysis. A popular technique to compute epistemic uncertainty is the Monte-Carlo (MC) dropout technique. From a network with MC dropout and a single input, multiple outputs can be sampled. Various methods can be used to obtain epistemic uncertainty maps from those multiple outputs. In the case of multi-class segmentation, the number of methods is even larger as epistemic uncertainty can be computed voxelwise per class or voxelwise per image. This paper highlights a systematic approach to define and quantitatively compare those methods in two different contexts: class-specific epistemic uncertainty maps (one value per image, voxel and class) and combined epistemic uncertainty maps (one value per image and voxel). We applied this quantitative analysis to a multi-class segmentation of the carotid artery lumen and vessel wall, on a multi-center, multi-scanner, multi-sequence dataset of (MR) images. We validated our analysis over 144 sets of hyperparameters of a model. Our main analysis considers the relationship between the order of the voxels sorted according to their epistemic uncertainty values and the misclassification of the prediction. Under this consideration, the comparison of combined uncertainty maps reveals that the multi-class entropy and the multi-class mutual information statistically out-perform the other combined uncertainty maps under study. In a class-specific scenario, the one-versus-all entropy statistically out-performs the class-wise entropy, the class-wise variance and the one versus all mutual information. The class-wise entropy statistically out-performs the other class-specific uncertainty maps in terms of calibration. We made a python package available to reproduce our analysis on different data and tasks.
Automated Segmentation and Volume Measurement of Intracranial Carotid Artery Calcification on Non-Contrast CT
Jul 20, 2021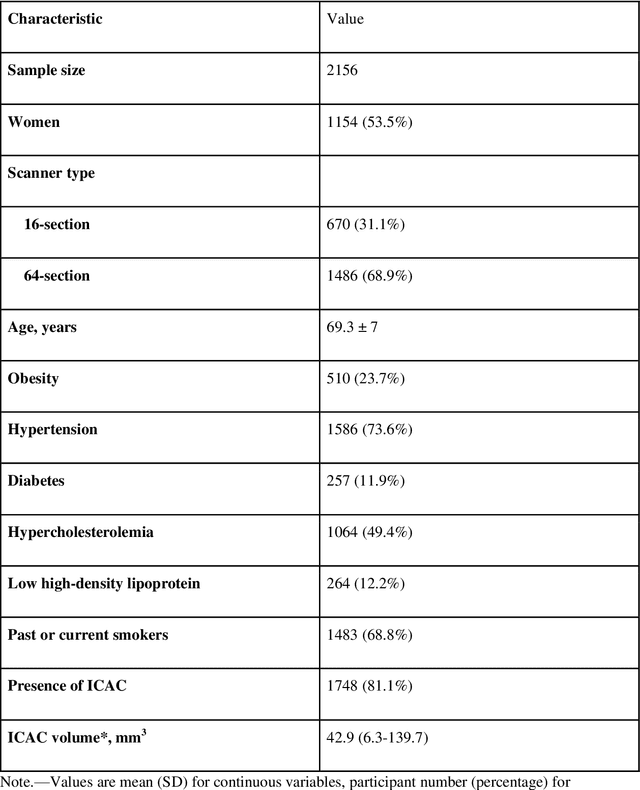
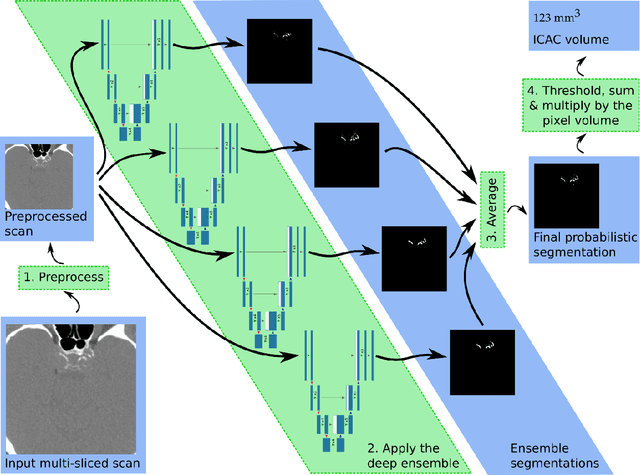
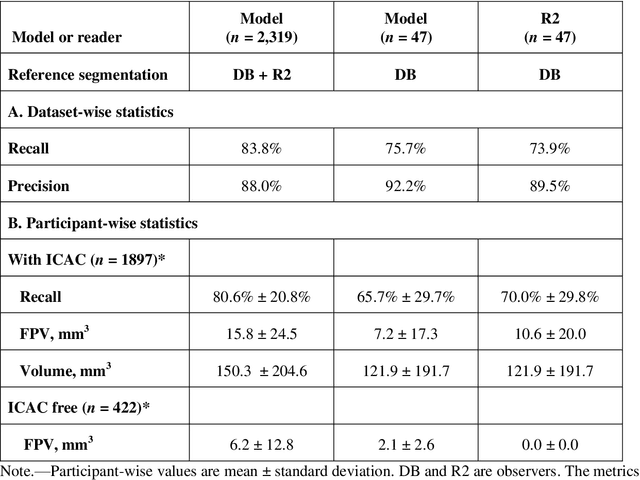
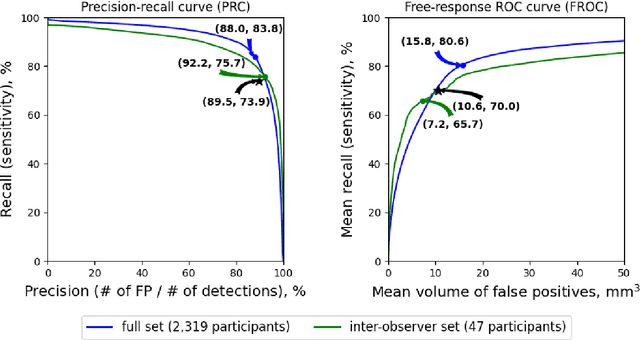
Abstract:Purpose: To evaluate a fully-automated deep-learning-based method for assessment of intracranial carotid artery calcification (ICAC). Methods: Two observers manually delineated ICAC in non-contrast CT scans of 2,319 participants (mean age 69 (SD 7) years; 1154 women) of the Rotterdam Study, prospectively collected between 2003 and 2006. These data were used to retrospectively develop and validate a deep-learning-based method for automated ICAC delineation and volume measurement. To evaluate the method, we compared manual and automatic assessment (computed using ten-fold cross-validation) with respect to 1) the agreement with an independent observer's assessment (available in a random subset of 47 scans); 2) the accuracy in delineating ICAC as judged via blinded visual comparison by an expert; 3) the association with first stroke incidence from the scan date until 2012. All method performance metrics were computed using 10-fold cross-validation. Results: The automated delineation of ICAC reached sensitivity of 83.8% and positive predictive value (PPV) of 88%. The intraclass correlation between automatic and manual ICAC volume measures was 0.98 (95% CI: 0.97, 0.98; computed in the entire dataset). Measured between the assessments of independent observers, sensitivity was 73.9%, PPV was 89.5%, and intraclass correlation was 0.91 (95% CI: 0.84, 0.95; computed in the 47-scan subset). In the blinded visual comparisons, automatic delineations were more accurate than manual ones (p-value = 0.01). The association of ICAC volume with incident stroke was similarly strong for both automated (hazard ratio, 1.38 (95% CI: 1.12, 1.75) and manually measured volumes (hazard ratio, 1.48 (95% CI: 1.20, 1.87)). Conclusions: The developed model was capable of automated segmentation and volume quantification of ICAC with accuracy comparable to human experts.
Segmentation of Intracranial Arterial Calcification with Deeply Supervised Residual Dropout Networks
Jun 04, 2017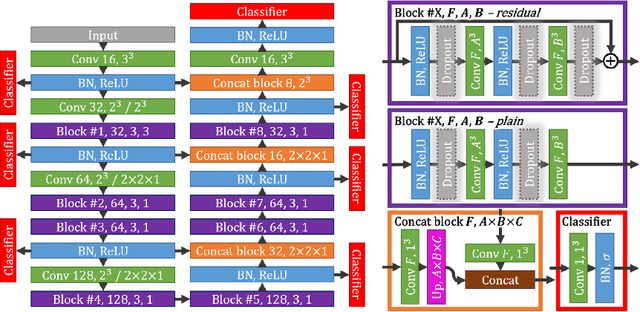
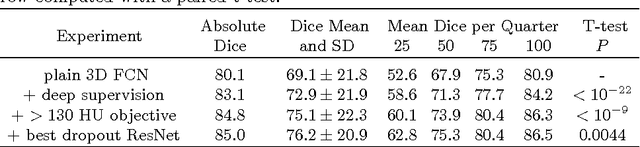
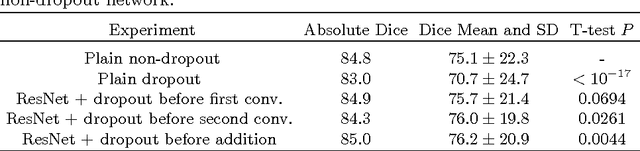
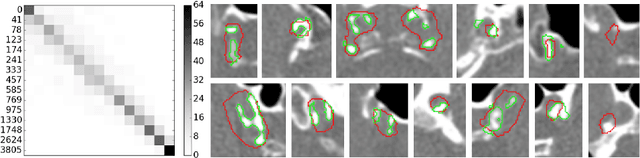
Abstract:Intracranial carotid artery calcification (ICAC) is a major risk factor for stroke, and might contribute to dementia and cognitive decline. Reliance on time-consuming manual annotation of ICAC hampers much demanded further research into the relationship between ICAC and neurological diseases. Automation of ICAC segmentation is therefore highly desirable, but difficult due to the proximity of the lesions to bony structures with a similar attenuation coefficient. In this paper, we propose a method for automatic segmentation of ICAC; the first to our knowledge. Our method is based on a 3D fully convolutional neural network that we extend with two regularization techniques. Firstly, we use deep supervision (hidden layers supervision) to encourage discriminative features in the hidden layers. Secondly, we augment the network with skip connections, as in the recently developed ResNet, and dropout layers, inserted in a way that skip connections circumvent them. We investigate the effect of skip connections and dropout. In addition, we propose a simple problem-specific modification of the network objective function that restricts the focus to the most important image regions and simplifies the optimization. We train and validate our model using 882 CT scans and test on 1,000. Our regularization techniques and objective improve the average Dice score by 7.1%, yielding an average Dice of 76.2% and 97.7% correlation between predicted ICAC volumes and manual annotations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge