M. Kamran Ikram
for the Alzheimer's Disease Neuroimaging Initiative
Automated Segmentation and Volume Measurement of Intracranial Carotid Artery Calcification on Non-Contrast CT
Jul 20, 2021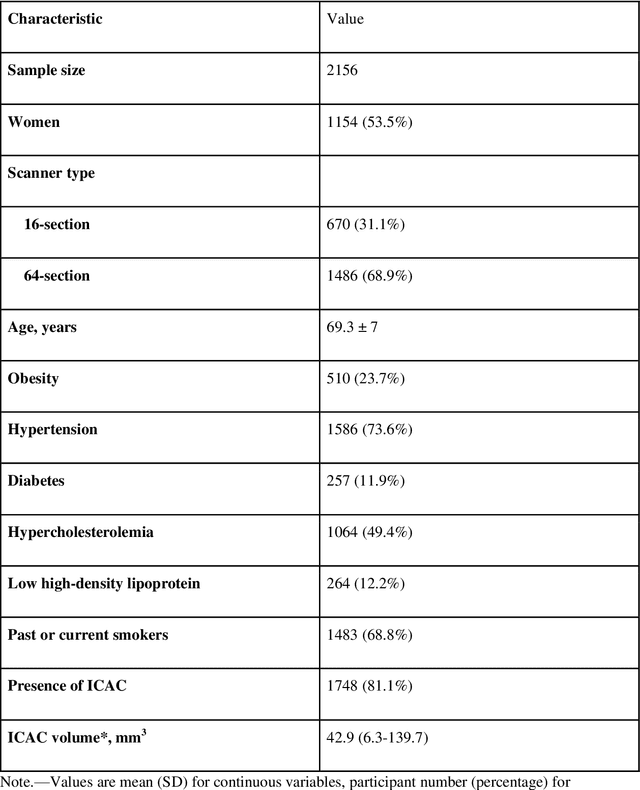
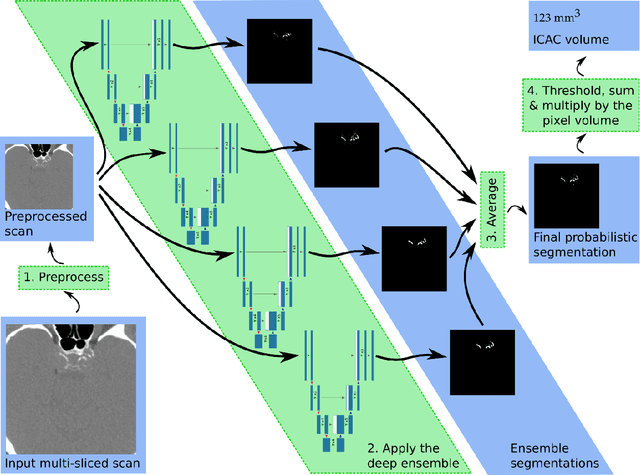
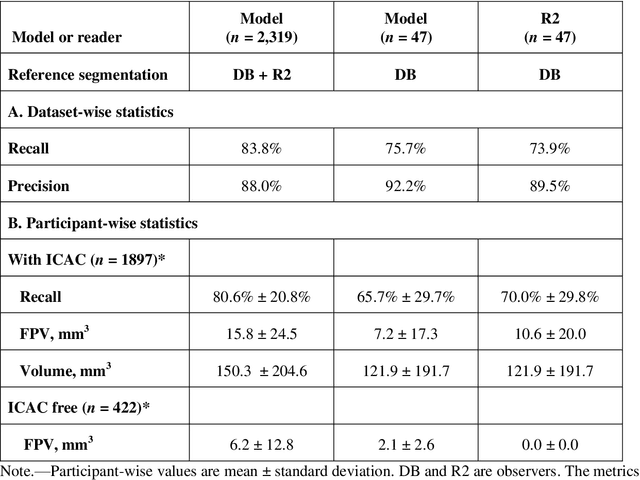
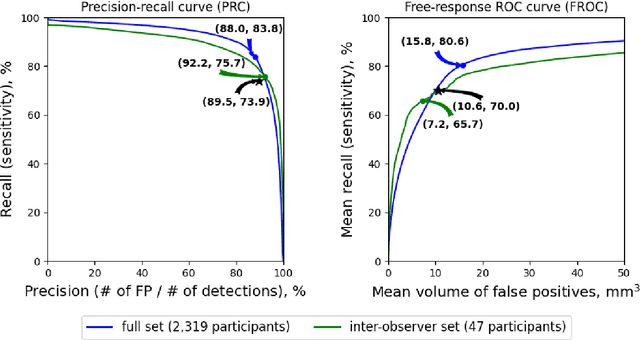
Abstract:Purpose: To evaluate a fully-automated deep-learning-based method for assessment of intracranial carotid artery calcification (ICAC). Methods: Two observers manually delineated ICAC in non-contrast CT scans of 2,319 participants (mean age 69 (SD 7) years; 1154 women) of the Rotterdam Study, prospectively collected between 2003 and 2006. These data were used to retrospectively develop and validate a deep-learning-based method for automated ICAC delineation and volume measurement. To evaluate the method, we compared manual and automatic assessment (computed using ten-fold cross-validation) with respect to 1) the agreement with an independent observer's assessment (available in a random subset of 47 scans); 2) the accuracy in delineating ICAC as judged via blinded visual comparison by an expert; 3) the association with first stroke incidence from the scan date until 2012. All method performance metrics were computed using 10-fold cross-validation. Results: The automated delineation of ICAC reached sensitivity of 83.8% and positive predictive value (PPV) of 88%. The intraclass correlation between automatic and manual ICAC volume measures was 0.98 (95% CI: 0.97, 0.98; computed in the entire dataset). Measured between the assessments of independent observers, sensitivity was 73.9%, PPV was 89.5%, and intraclass correlation was 0.91 (95% CI: 0.84, 0.95; computed in the 47-scan subset). In the blinded visual comparisons, automatic delineations were more accurate than manual ones (p-value = 0.01). The association of ICAC volume with incident stroke was similarly strong for both automated (hazard ratio, 1.38 (95% CI: 1.12, 1.75) and manually measured volumes (hazard ratio, 1.48 (95% CI: 1.20, 1.87)). Conclusions: The developed model was capable of automated segmentation and volume quantification of ICAC with accuracy comparable to human experts.
Analyzing the effect of APOE on Alzheimer's disease progression using an event-based model for stratified populations
Sep 15, 2020



Abstract:Alzheimer's disease (AD) is the most common form of dementia and is phenotypically heterogeneous. APOE is a triallelic gene which correlates with phenotypic heterogeneity in AD. In this work, we determined the effect of APOE alleles on the disease progression timeline of AD using a discriminative event-based model (DEBM). Since DEBM is a data-driven model, stratification into smaller disease subgroups would lead to more inaccurate models as compared to fitting the model on the entire dataset. Hence our secondary aim is to propose and evaluate novel approaches in which we split the different steps of DEBM into group-aspecific and group-specific parts, where the entire dataset is used to train the group-aspecific parts and only the data from a specific group is used to train the group-specific parts of the DEBM. We performed simulation experiments to benchmark the accuracy of the proposed approaches and to select the optimal approach. Subsequently, the chosen approach was applied to the baseline data of 417 cognitively normal, 235 mild cognitively impaired who convert to AD within 3 years, and 342 AD patients from the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset to gain new insights into the effect of APOE carriership on the disease progression timeline of AD. The presented models could aid understanding of the disease, and in selecting homogeneous group of presymptomatic subjects at-risk of developing symptoms for clinical trials.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge