Christian Payer
Efficient Multi-Organ Segmentation Using SpatialConfiguration-Net with Low GPU Memory Requirements
Nov 26, 2021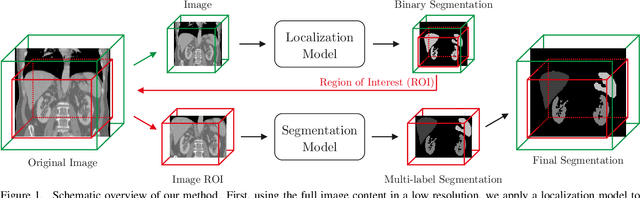

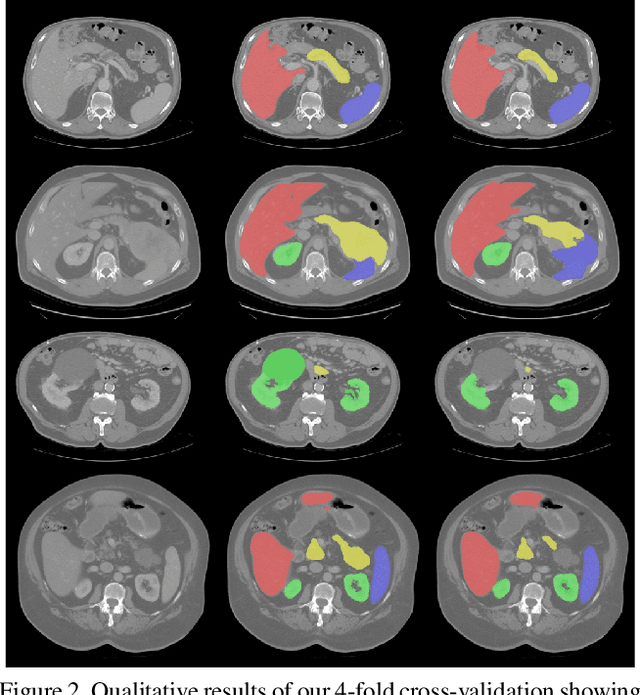
Abstract:Even though many semantic segmentation methods exist that are able to perform well on many medical datasets, often, they are not designed for direct use in clinical practice. The two main concerns are generalization to unseen data with a different visual appearance, e.g., images acquired using a different scanner, and efficiency in terms of computation time and required Graphics Processing Unit (GPU) memory. In this work, we employ a multi-organ segmentation model based on the SpatialConfiguration-Net (SCN), which integrates prior knowledge of the spatial configuration among the labelled organs to resolve spurious responses in the network outputs. Furthermore, we modified the architecture of the segmentation model to reduce its memory footprint as much as possible without drastically impacting the quality of the predictions. Lastly, we implemented a minimal inference script for which we optimized both, execution time and required GPU memory.
Modeling Annotation Uncertainty with Gaussian Heatmaps in Landmark Localization
Sep 21, 2021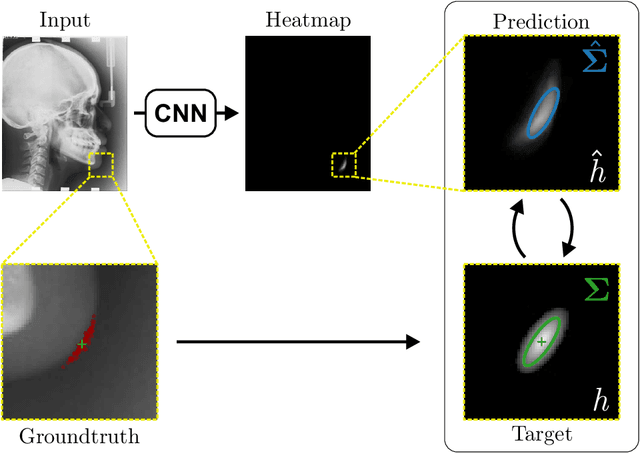
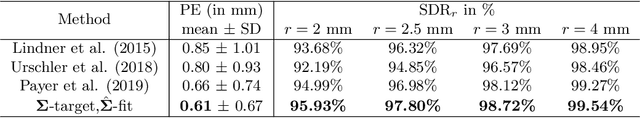
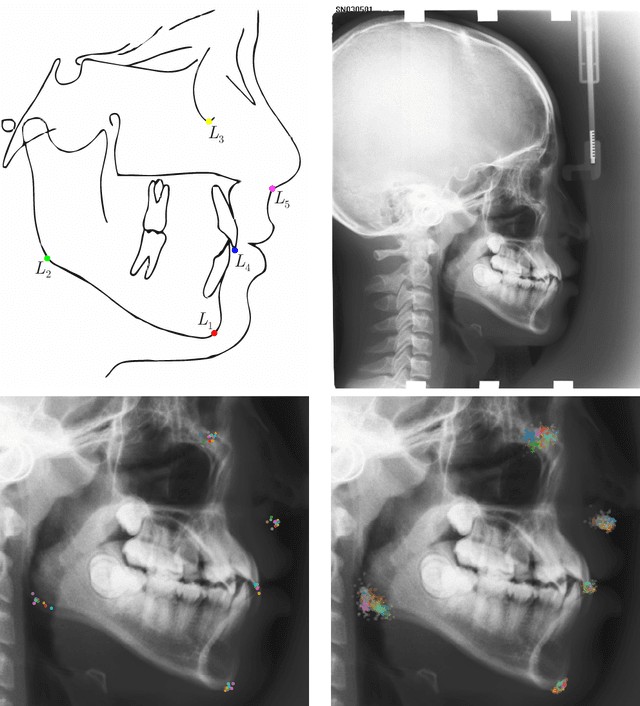
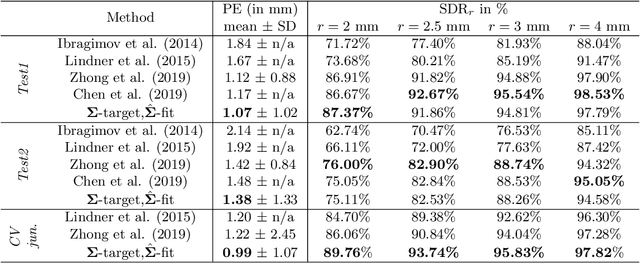
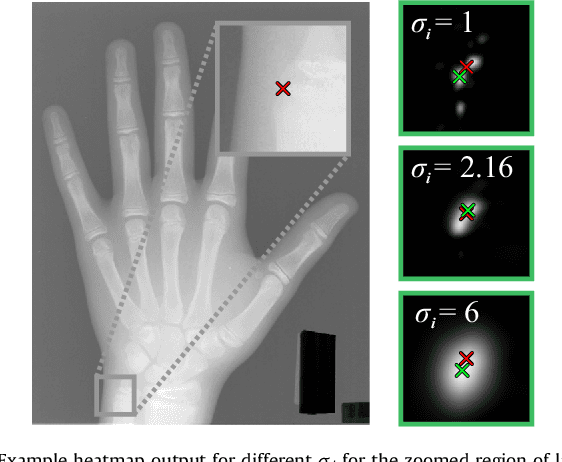
Abstract:In landmark localization, due to ambiguities in defining their exact position, landmark annotations may suffer from large observer variabilities, which result in uncertain annotations. To model the annotation ambiguities of the training dataset, we propose to learn anisotropic Gaussian parameters modeling the shape of the target heatmap during optimization. Furthermore, our method models the prediction uncertainty of individual samples by fitting anisotropic Gaussian functions to the predicted heatmaps during inference. Besides state-of-the-art results, our experiments on datasets of hand radiographs and lateral cephalograms also show that Gaussian functions are correlated with both localization accuracy and observer variability. As a final experiment, we show the importance of integrating the uncertainty into decision making by measuring the influence of the predicted location uncertainty on the classification of anatomical abnormalities in lateral cephalograms.
Inferring the 3D Standing Spine Posture from 2D Radiographs
Jul 13, 2020



Abstract:The treatment of degenerative spinal disorders requires an understanding of the individual spinal anatomy and curvature in 3D. An upright spinal pose (i.e. standing) under natural weight bearing is crucial for such bio-mechanical analysis. 3D volumetric imaging modalities (e.g. CT and MRI) are performed in patients lying down. On the other hand, radiographs are captured in an upright pose, but result in 2D projections. This work aims to integrate the two realms, i.e. it combines the upright spinal curvature from radiographs with the 3D vertebral shape from CT imaging for synthesizing an upright 3D model of spine, loaded naturally. Specifically, we propose a novel neural network architecture working vertebra-wise, termed \emph{TransVert}, which takes orthogonal 2D radiographs and infers the spine's 3D posture. We validate our architecture on digitally reconstructed radiographs, achieving a 3D reconstruction Dice of $95.52\%$, indicating an almost perfect 2D-to-3D domain translation. Deploying our model on clinical radiographs, we successfully synthesise full-3D, upright, patient-specific spine models for the first time.
Variational Inference and Bayesian CNNs for Uncertainty Estimation in Multi-Factorial Bone Age Prediction
Feb 25, 2020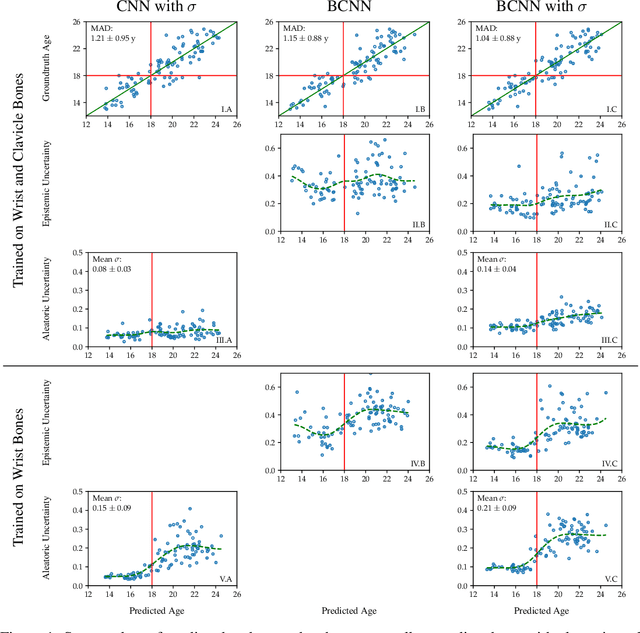
Abstract:Additionally to the extensive use in clinical medicine, biological age (BA) in legal medicine is used to assess unknown chronological age (CA) in applications where identification documents are not available. Automatic methods for age estimation proposed in the literature are predicting point estimates, which can be misleading without the quantification of predictive uncertainty. In our multi-factorial age estimation method from MRI data, we used the Variational Inference approach to estimate the uncertainty of a Bayesian CNN model. Distinguishing model uncertainty from data uncertainty, we interpreted data uncertainty as biological variation, i.e. the range of possible CA of subjects having the same BA.
VerSe: A Vertebrae Labelling and Segmentation Benchmark
Jan 24, 2020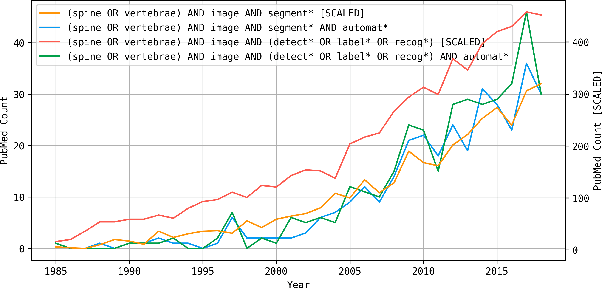
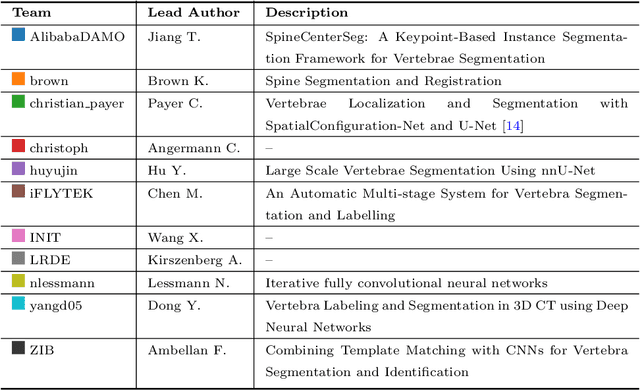
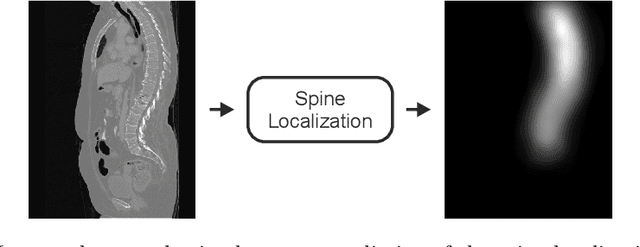
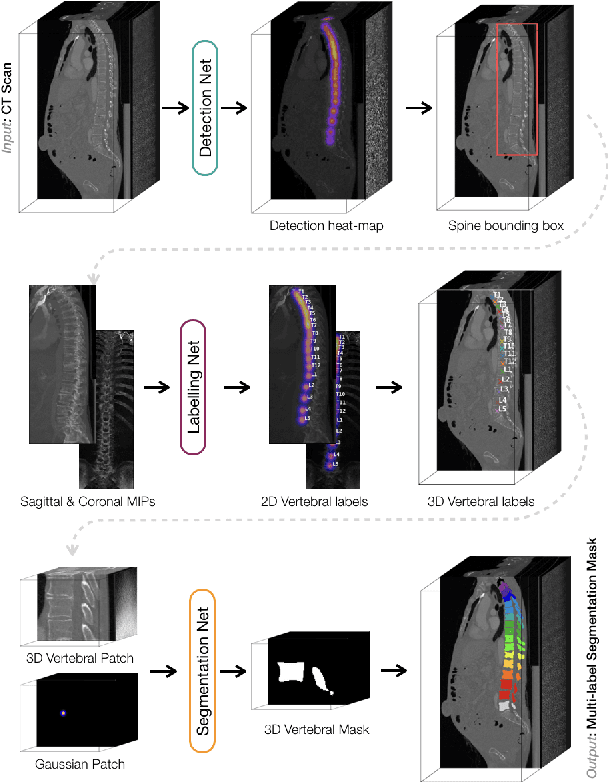
Abstract:In this paper we report the challenge set-up and results of the Large Scale Vertebrae Segmentation Challenge (VerSe) organized in conjunction with the MICCAI 2019. The challenge consisted of two tasks, vertebrae labelling and vertebrae segmentation. For this a total of 160 multidetector CT scan cohort closely resembling clinical setting was prepared and was annotated at a voxel-level by a human-machine hybrid algorithm. In this paper we also present the annotation protocol and the algorithm that aided the medical experts in the annotation process. Eleven fully automated algorithms were benchmarked on this data with the best performing algorithm achieving a vertebrae identification rate of 95% and a Dice coefficient of 90%. VerSe'19 is an open-call challenge at its image data along with the annotations and evaluation tools will continue to be publicly accessible through its online portal.
Integrating Spatial Configuration into Heatmap Regression Based CNNs for Landmark Localization
Aug 02, 2019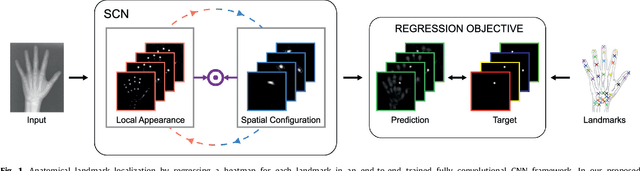
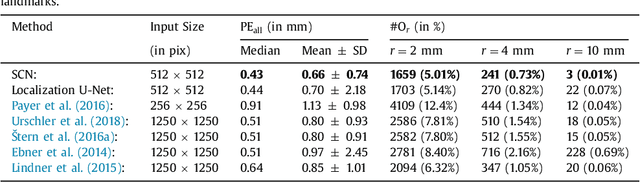
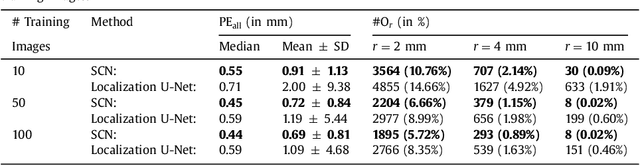
Abstract:In many medical image analysis applications, often only a limited amount of training data is available, which makes training of convolutional neural networks (CNNs) challenging. In this work on anatomical landmark localization, we propose a CNN architecture that learns to split the localization task into two simpler sub-problems, reducing the need for large training datasets. Our fully convolutional SpatialConfiguration-Net (SCN) dedicates one component to locally accurate but ambiguous candidate predictions, while the other component improves robustness to ambiguities by incorporating the spatial configuration of landmarks. In our experimental evaluation, we show that the proposed SCN outperforms related methods in terms of landmark localization error on size-limited datasets.
Evaluation of Algorithms for Multi-Modality Whole Heart Segmentation: An Open-Access Grand Challenge
Feb 21, 2019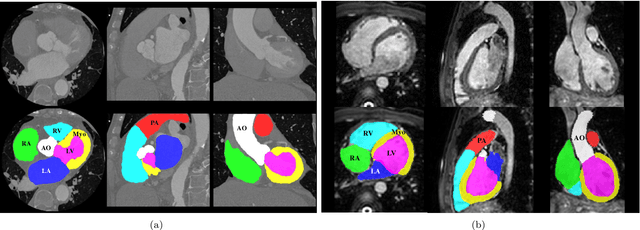

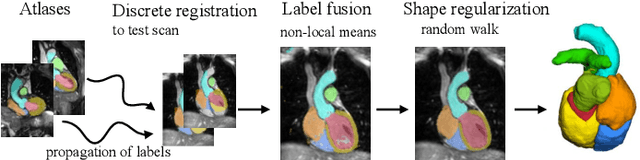
Abstract:Knowledge of whole heart anatomy is a prerequisite for many clinical applications. Whole heart segmentation (WHS), which delineates substructures of the heart, can be very valuable for modeling and analysis of the anatomy and functions of the heart. However, automating this segmentation can be arduous due to the large variation of the heart shape, and different image qualities of the clinical data. To achieve this goal, a set of training data is generally needed for constructing priors or for training. In addition, it is difficult to perform comparisons between different methods, largely due to differences in the datasets and evaluation metrics used. This manuscript presents the methodologies and evaluation results for the WHS algorithms selected from the submissions to the Multi-Modality Whole Heart Segmentation (MM-WHS) challenge, in conjunction with MICCAI 2017. The challenge provides 120 three-dimensional cardiac images covering the whole heart, including 60 CT and 60 MRI volumes, all acquired in clinical environments with manual delineation. Ten algorithms for CT data and eleven algorithms for MRI data, submitted from twelve groups, have been evaluated. The results show that many of the deep learning (DL) based methods achieved high accuracy, even though the number of training datasets was limited. A number of them also reported poor results in the blinded evaluation, probably due to overfitting in their training. The conventional algorithms, mainly based on multi-atlas segmentation, demonstrated robust and stable performance, even though the accuracy is not as good as the best DL method in CT segmentation. The challenge, including the provision of the annotated training data and the blinded evaluation for submitted algorithms on the test data, continues as an ongoing benchmarking resource via its homepage (\url{www.sdspeople.fudan.edu.cn/zhuangxiahai/0/mmwhs/}).
Instance Segmentation and Tracking with Cosine Embeddings and Recurrent Hourglass Networks
Jul 30, 2018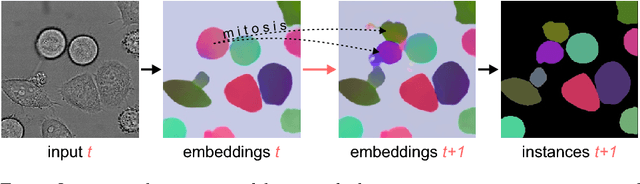
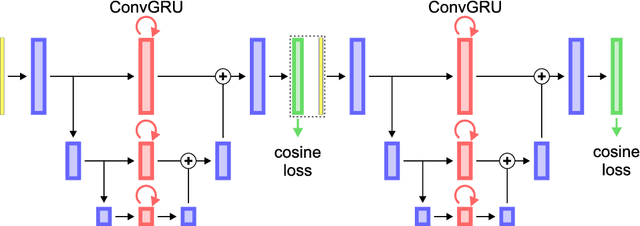
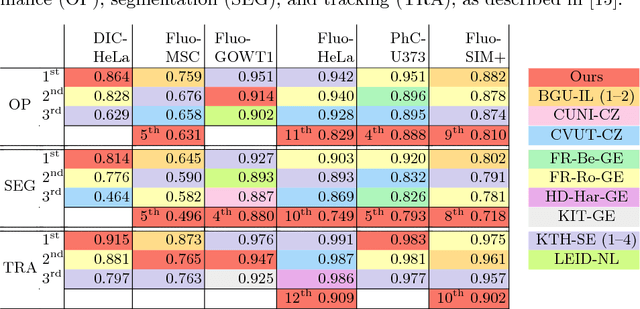
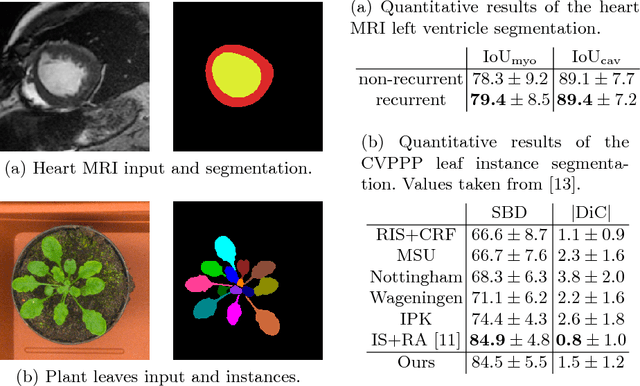
Abstract:Different to semantic segmentation, instance segmentation assigns unique labels to each individual instance of the same class. In this work, we propose a novel recurrent fully convolutional network architecture for tracking such instance segmentations over time. The network architecture incorporates convolutional gated recurrent units (ConvGRU) into a stacked hourglass network to utilize temporal video information. Furthermore, we train the network with a novel embedding loss based on cosine similarities, such that the network predicts unique embeddings for every instance throughout videos. Afterwards, these embeddings are clustered among subsequent video frames to create the final tracked instance segmentations. We evaluate the recurrent hourglass network by segmenting left ventricles in MR videos of the heart, where it outperforms a network that does not incorporate video information. Furthermore, we show applicability of the cosine embedding loss for segmenting leaf instances on still images of plants. Finally, we evaluate the framework for instance segmentation and tracking on six datasets of the ISBI celltracking challenge, where it shows state-of-the-art performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge