Changyan He
Electromagnets Under the Table: an Unobtrusive Magnetic Navigation System for Microsurgery
Aug 23, 2023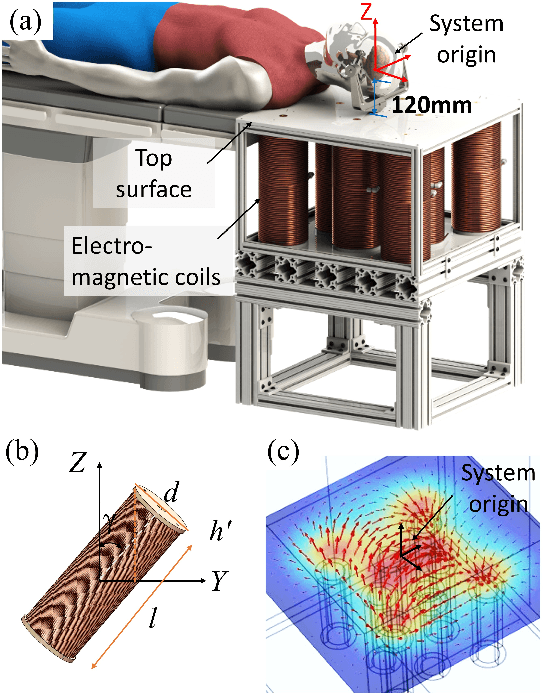
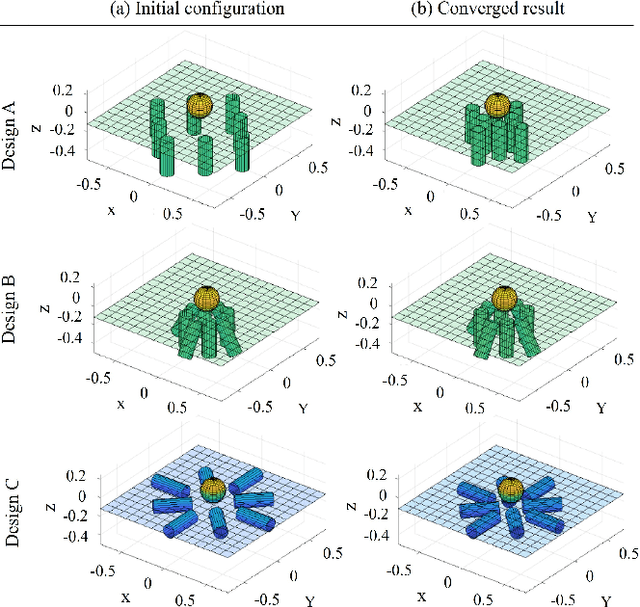
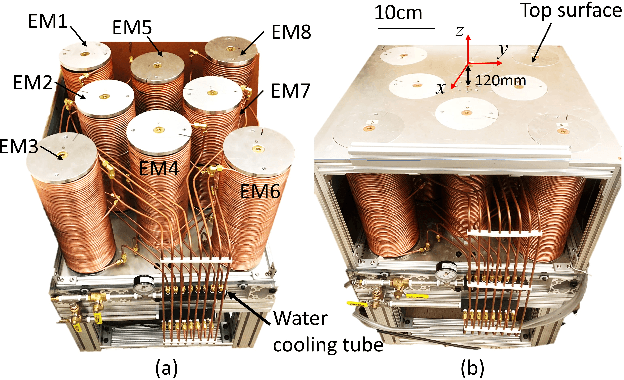
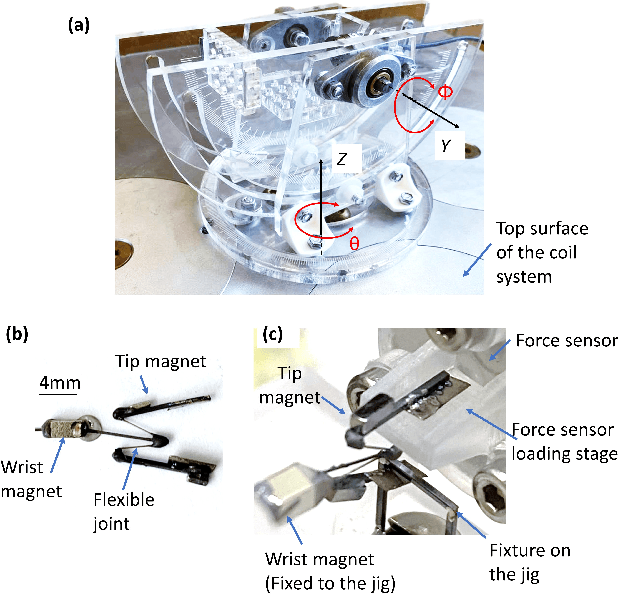
Abstract:Miniature magnetic tools have the potential to enable minimally invasive surgical techniques to be applied to space-restricted surgical procedures in areas such as neurosurgery. However, typical magnetic navigation systems, which create the magnetic fields to drive such tools, either cannot generate large enough fields, or surround the patient in a way that obstructs surgeon access to the patient. This paper introduces the design of a magnetic navigation system with eight electromagnets arranged completely under the operating table, to endow the system with maximal workspace accessibility, which allows the patient to lie down on the top surface of the system without any constraints. The found optimal geometric layout of the electromagnets maximizes the field strength and uniformity over a reasonable neurosurgical operating volume. The system can generate non-uniform magnetic fields up to 38 mT along the x and y axes and 47 mT along the z axis at a working distance of 120 mm away from the actuation system workbench, deep enough to deploy magnetic microsurgical tools in the brain. The forces which can be exerted on millimeter-scale magnets used in prototype neurosurgical tools are validated experimentally. Due to its large workspace, this system could be used to control milli-robots in a variety of surgical applications.
Spotlight-based 3D Instrument Guidance for Retinal Surgery
Dec 11, 2020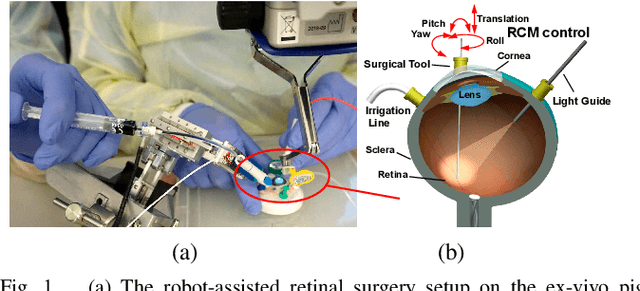
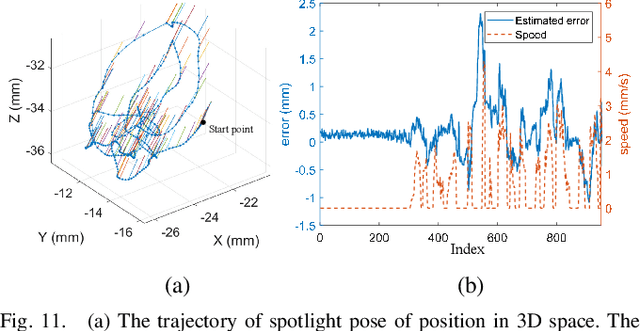
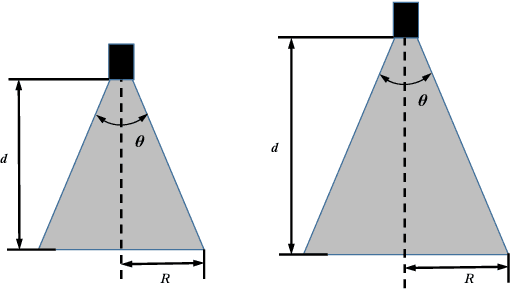
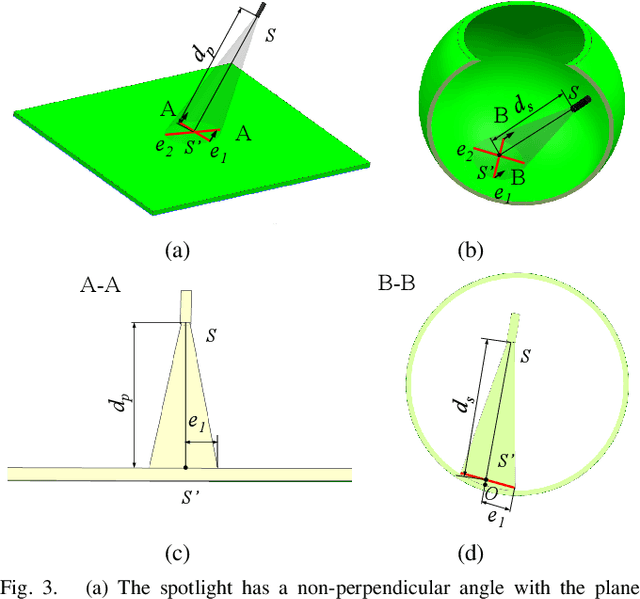
Abstract:Retinal surgery is a complex activity that can be challenging for a surgeon to perform effectively and safely. Image guided robot-assisted surgery is one of the promising solutions that bring significant surgical enhancement in treatment outcome and reduce the physical limitations of human surgeons. In this paper, we demonstrate a novel method for 3D guidance of the instrument based on the projection of spotlight in the single microscope images. The spotlight projection mechanism is firstly analyzed and modeled with a projection on both a plane and a sphere surface. To test the feasibility of the proposed method, a light fiber is integrated into the instrument which is driven by the Steady-Hand Eye Robot (SHER). The spot of light is segmented and tracked on a phantom retina using the proposed algorithm. The static calibration and dynamic test results both show that the proposed method can easily archive 0.5 mm of tip-to-surface distance which is within the clinically acceptable accuracy for intraocular visual guidance.
Autonomously Navigating a Surgical Tool Inside the Eye by Learning from Demonstration
Nov 16, 2020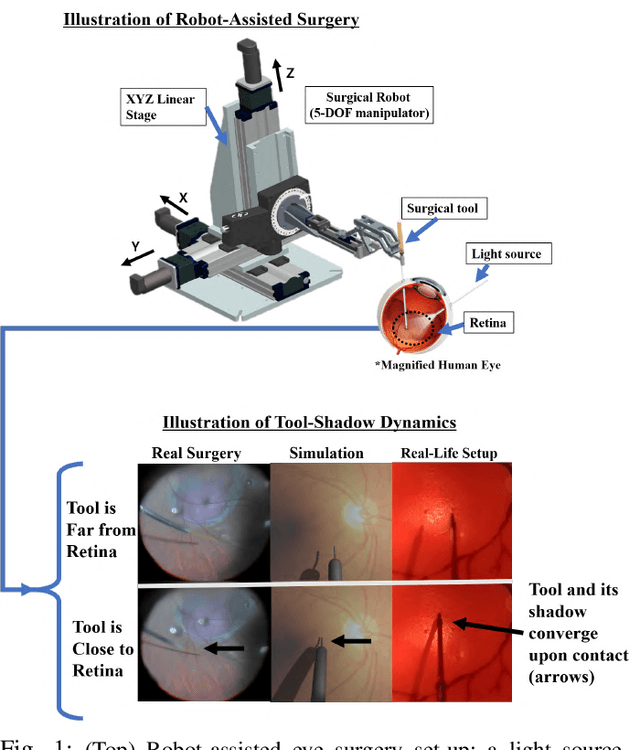
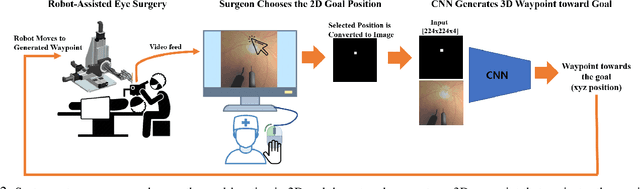
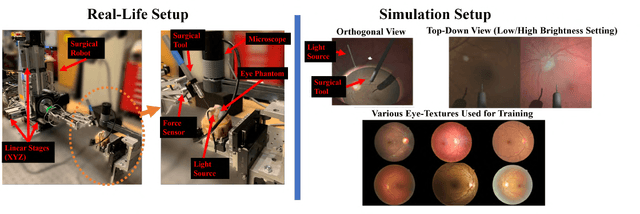
Abstract:A fundamental challenge in retinal surgery is safely navigating a surgical tool to a desired goal position on the retinal surface while avoiding damage to surrounding tissues, a procedure that typically requires tens-of-microns accuracy. In practice, the surgeon relies on depth-estimation skills to localize the tool-tip with respect to the retina in order to perform the tool-navigation task, which can be prone to human error. To alleviate such uncertainty, prior work has introduced ways to assist the surgeon by estimating the tool-tip distance to the retina and providing haptic or auditory feedback. However, automating the tool-navigation task itself remains unsolved and largely unexplored. Such a capability, if reliably automated, could serve as a building block to streamline complex procedures and reduce the chance for tissue damage. Towards this end, we propose to automate the tool-navigation task by learning to mimic expert demonstrations of the task. Specifically, a deep network is trained to imitate expert trajectories toward various locations on the retina based on recorded visual servoing to a given goal specified by the user. The proposed autonomous navigation system is evaluated in simulation and in physical experiments using a silicone eye phantom. We show that the network can reliably navigate a needle surgical tool to various desired locations within 137 microns accuracy in physical experiments and 94 microns in simulation on average, and generalizes well to unseen situations such as in the presence of auxiliary surgical tools, variable eye backgrounds, and brightness conditions.
Sclera Force Control in Robot-assisted Eye Surgery: Adaptive Force Control vs. Auditory Feedback
Jan 10, 2019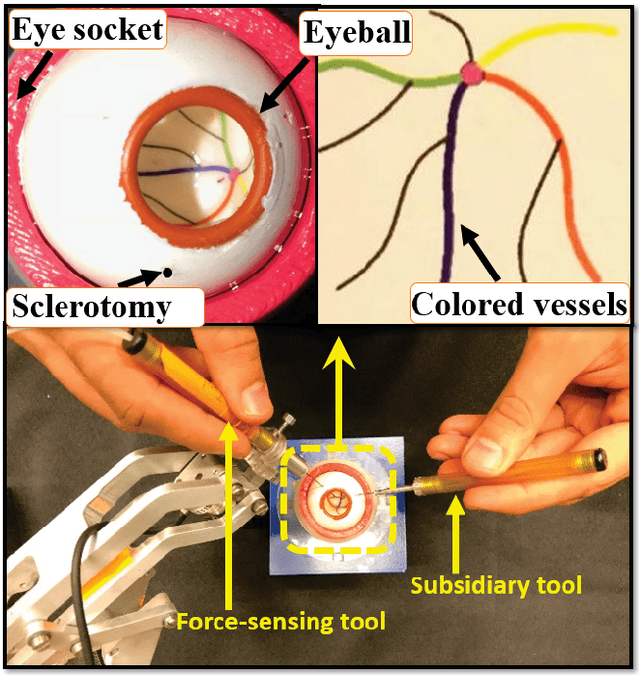
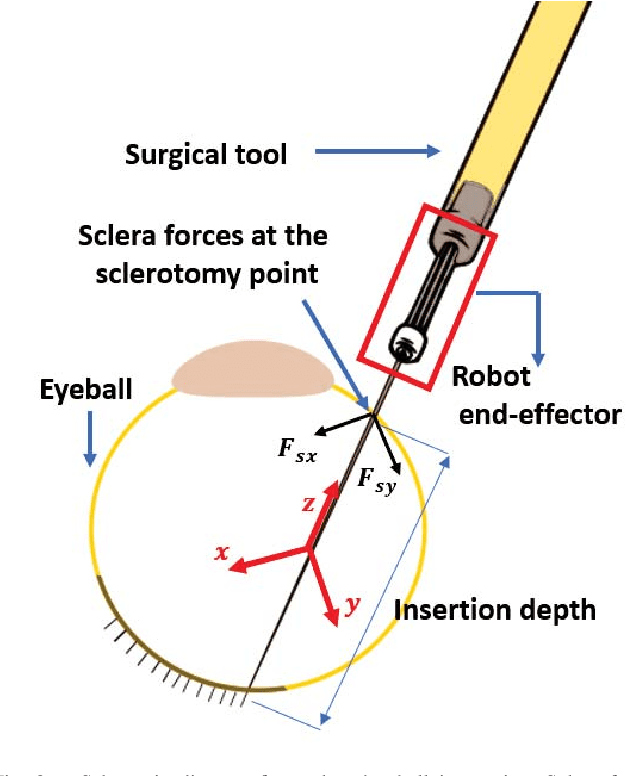
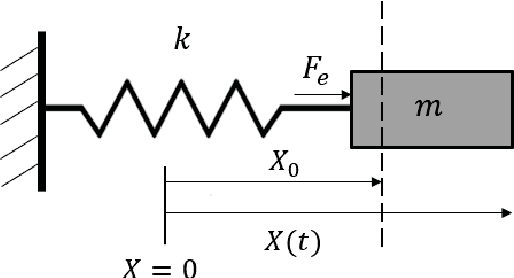
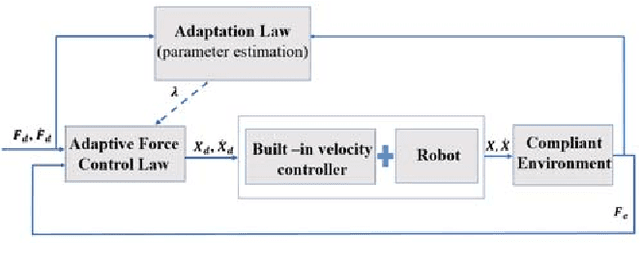
Abstract:Surgeon hand tremor limits human capability during microsurgical procedures such as those that treat the eye. In contrast, elimination of hand tremor through the introduction of microsurgical robots diminishes the surgeon's tactile perception of useful and familiar tool-to-sclera forces. While the large mass and inertia of eye surgical robot prevents surgeon microtremor, loss of perception of small scleral forces may put the sclera at risk of injury. In this paper, we have applied and compared two different methods to assure the safety of sclera tissue during robot-assisted eye surgery. In the active control method, an adaptive force control strategy is implemented on the Steady-Hand Eye Robot in order to control the magnitude of scleral forces when they exceed safe boundaries. This autonomous force compensation is then compared to a passive force control method in which the surgeon performs manual adjustments in response to the provided audio feedback proportional to the magnitude of sclera force. A pilot study with three users indicate that the active control method is potentially more efficient.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge