Adriano Lucieri
LD-ViCE: Latent Diffusion Model for Video Counterfactual Explanations
Sep 10, 2025



Abstract:Video-based AI systems are increasingly adopted in safety-critical domains such as autonomous driving and healthcare. However, interpreting their decisions remains challenging due to the inherent spatiotemporal complexity of video data and the opacity of deep learning models. Existing explanation techniques often suffer from limited temporal coherence, insufficient robustness, and a lack of actionable causal insights. Current counterfactual explanation methods typically do not incorporate guidance from the target model, reducing semantic fidelity and practical utility. We introduce Latent Diffusion for Video Counterfactual Explanations (LD-ViCE), a novel framework designed to explain the behavior of video-based AI models. Compared to previous approaches, LD-ViCE reduces the computational costs of generating explanations by operating in latent space using a state-of-the-art diffusion model, while producing realistic and interpretable counterfactuals through an additional refinement step. Our experiments demonstrate the effectiveness of LD-ViCE across three diverse video datasets, including EchoNet-Dynamic (cardiac ultrasound), FERV39k (facial expression), and Something-Something V2 (action recognition). LD-ViCE outperforms a recent state-of-the-art method, achieving an increase in R2 score of up to 68% while reducing inference time by half. Qualitative analysis confirms that LD-ViCE generates semantically meaningful and temporally coherent explanations, offering valuable insights into the target model behavior. LD-ViCE represents a valuable step toward the trustworthy deployment of AI in safety-critical domains.
Unifying VXAI: A Systematic Review and Framework for the Evaluation of Explainable AI
Jun 18, 2025Abstract:Modern AI systems frequently rely on opaque black-box models, most notably Deep Neural Networks, whose performance stems from complex architectures with millions of learned parameters. While powerful, their complexity poses a major challenge to trustworthiness, particularly due to a lack of transparency. Explainable AI (XAI) addresses this issue by providing human-understandable explanations of model behavior. However, to ensure their usefulness and trustworthiness, such explanations must be rigorously evaluated. Despite the growing number of XAI methods, the field lacks standardized evaluation protocols and consensus on appropriate metrics. To address this gap, we conduct a systematic literature review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and introduce a unified framework for the eValuation of XAI (VXAI). We identify 362 relevant publications and aggregate their contributions into 41 functionally similar metric groups. In addition, we propose a three-dimensional categorization scheme spanning explanation type, evaluation contextuality, and explanation quality desiderata. Our framework provides the most comprehensive and structured overview of VXAI to date. It supports systematic metric selection, promotes comparability across methods, and offers a flexible foundation for future extensions.
Discovering Concept Directions from Diffusion-based Counterfactuals via Latent Clustering
May 11, 2025Abstract:Concept-based explanations have emerged as an effective approach within Explainable Artificial Intelligence, enabling interpretable insights by aligning model decisions with human-understandable concepts. However, existing methods rely on computationally intensive procedures and struggle to efficiently capture complex, semantic concepts. Recently, the Concept Discovery through Latent Diffusion-based Counterfactual Trajectories (CDCT) framework, introduced by Varshney et al. (2025), attempts to identify concepts via dimension-wise traversal of the latent space of a Variational Autoencoder trained on counterfactual trajectories. Extending the CDCT framework, this work introduces Concept Directions via Latent Clustering (CDLC), which extracts global, class-specific concept directions by clustering latent difference vectors derived from factual and diffusion-generated counterfactual image pairs. CDLC substantially reduces computational complexity by eliminating the exhaustive latent dimension traversal required in CDCT and enables the extraction of multidimensional semantic concepts encoded across the latent dimensions. This approach is validated on a real-world skin lesion dataset, demonstrating that the extracted concept directions align with clinically recognized dermoscopic features and, in some cases, reveal dataset-specific biases or unknown biomarkers. These results highlight that CDLC is interpretable, scalable, and applicable across high-stakes domains and diverse data modalities.
Generating Counterfactual Trajectories with Latent Diffusion Models for Concept Discovery
Apr 16, 2024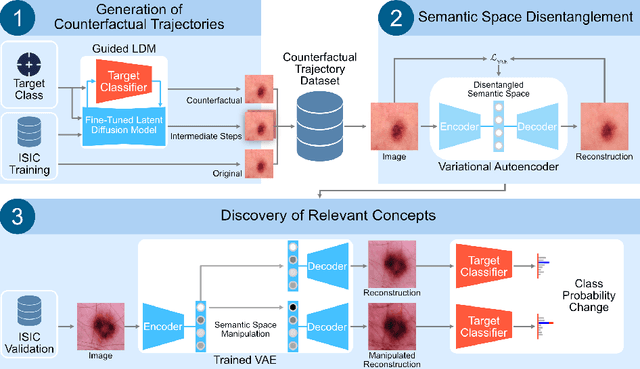
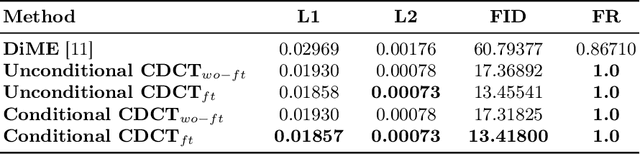
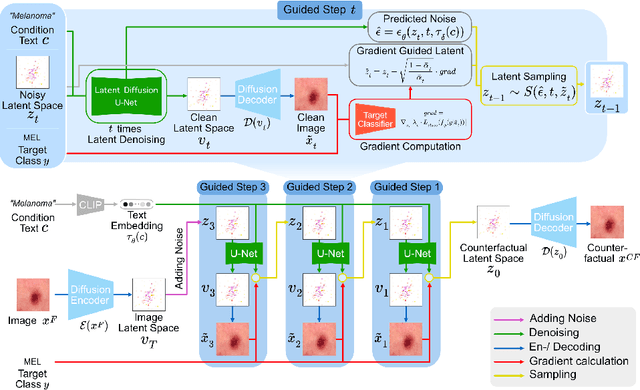
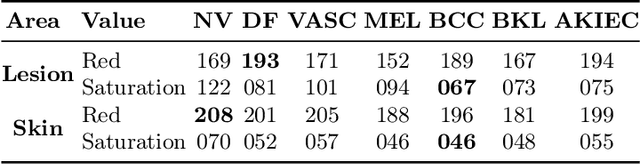
Abstract:Trustworthiness is a major prerequisite for the safe application of opaque deep learning models in high-stakes domains like medicine. Understanding the decision-making process not only contributes to fostering trust but might also reveal previously unknown decision criteria of complex models that could advance the state of medical research. The discovery of decision-relevant concepts from black box models is a particularly challenging task. This study proposes Concept Discovery through Latent Diffusion-based Counterfactual Trajectories (CDCT), a novel three-step framework for concept discovery leveraging the superior image synthesis capabilities of diffusion models. In the first step, CDCT uses a Latent Diffusion Model (LDM) to generate a counterfactual trajectory dataset. This dataset is used to derive a disentangled representation of classification-relevant concepts using a Variational Autoencoder (VAE). Finally, a search algorithm is applied to identify relevant concepts in the disentangled latent space. The application of CDCT to a classifier trained on the largest public skin lesion dataset revealed not only the presence of several biases but also meaningful biomarkers. Moreover, the counterfactuals generated within CDCT show better FID scores than those produced by a previously established state-of-the-art method, while being 12 times more resource-efficient. Unsupervised concept discovery holds great potential for the application of trustworthy AI and the further development of human knowledge in various domains. CDCT represents a further step in this direction.
Privacy Meets Explainability: A Comprehensive Impact Benchmark
Nov 08, 2022Abstract:Since the mid-10s, the era of Deep Learning (DL) has continued to this day, bringing forth new superlatives and innovations each year. Nevertheless, the speed with which these innovations translate into real applications lags behind this fast pace. Safety-critical applications, in particular, underlie strict regulatory and ethical requirements which need to be taken care of and are still active areas of debate. eXplainable AI (XAI) and privacy-preserving machine learning (PPML) are both crucial research fields, aiming at mitigating some of the drawbacks of prevailing data-hungry black-box models in DL. Despite brisk research activity in the respective fields, no attention has yet been paid to their interaction. This work is the first to investigate the impact of private learning techniques on generated explanations for DL-based models. In an extensive experimental analysis covering various image and time series datasets from multiple domains, as well as varying privacy techniques, XAI methods, and model architectures, the effects of private training on generated explanations are studied. The findings suggest non-negligible changes in explanations through the introduction of privacy. Apart from reporting individual effects of PPML on XAI, the paper gives clear recommendations for the choice of techniques in real applications. By unveiling the interdependencies of these pivotal technologies, this work is a first step towards overcoming the remaining hurdles for practically applicable AI in safety-critical domains.
Revisiting the Shape-Bias of Deep Learning for Dermoscopic Skin Lesion Classification
Jun 13, 2022


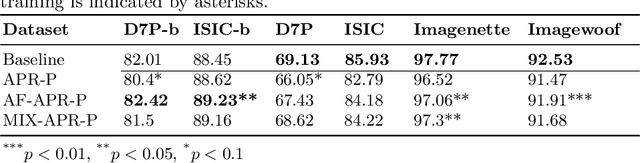
Abstract:It is generally believed that the human visual system is biased towards the recognition of shapes rather than textures. This assumption has led to a growing body of work aiming to align deep models' decision-making processes with the fundamental properties of human vision. The reliance on shape features is primarily expected to improve the robustness of these models under covariate shift. In this paper, we revisit the significance of shape-biases for the classification of skin lesion images. Our analysis shows that different skin lesion datasets exhibit varying biases towards individual image features. Interestingly, despite deep feature extractors being inclined towards learning entangled features for skin lesion classification, individual features can still be decoded from this entangled representation. This indicates that these features are still represented in the learnt embedding spaces of the models, but not used for classification. In addition, the spectral analysis of different datasets shows that in contrast to common visual recognition, dermoscopic skin lesion classification, by nature, is reliant on complex feature combinations beyond shape-bias. As a natural consequence, shifting away from the prevalent desire of shape-biasing models can even improve skin lesion classifiers in some cases.
ExAID: A Multimodal Explanation Framework for Computer-Aided Diagnosis of Skin Lesions
Jan 04, 2022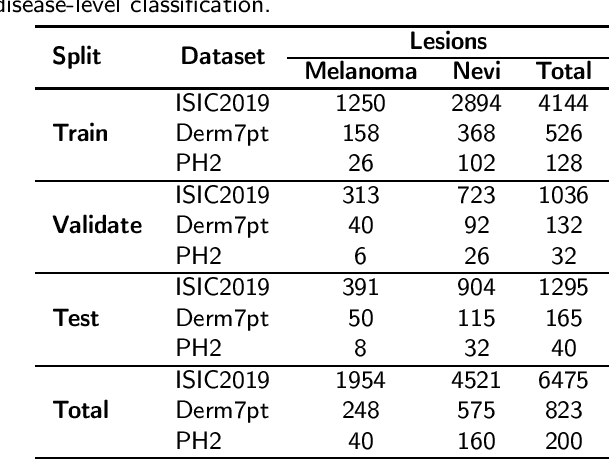
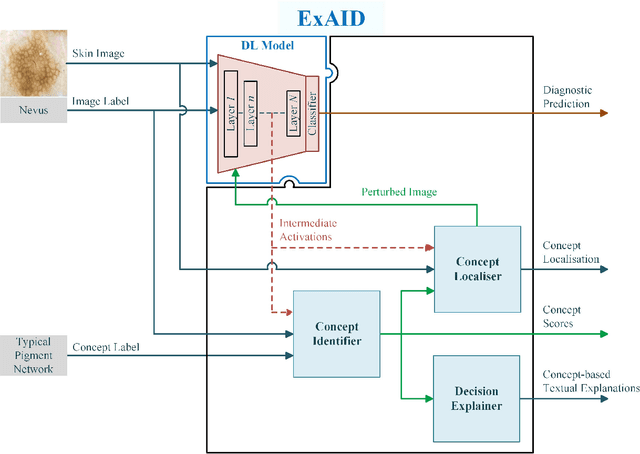
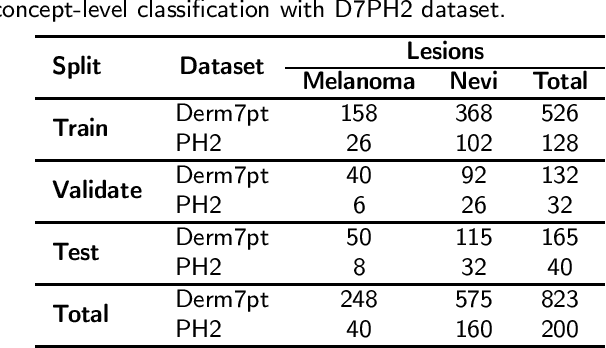
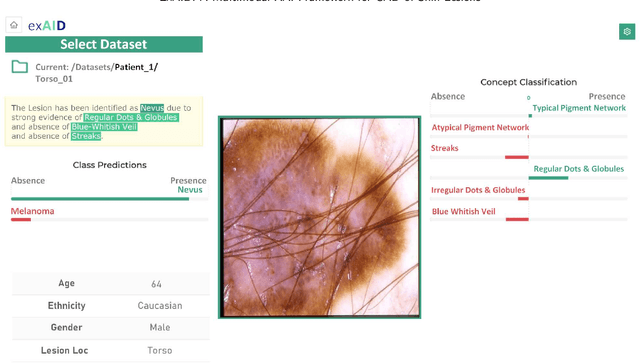
Abstract:One principal impediment in the successful deployment of AI-based Computer-Aided Diagnosis (CAD) systems in clinical workflows is their lack of transparent decision making. Although commonly used eXplainable AI methods provide some insight into opaque algorithms, such explanations are usually convoluted and not readily comprehensible except by highly trained experts. The explanation of decisions regarding the malignancy of skin lesions from dermoscopic images demands particular clarity, as the underlying medical problem definition is itself ambiguous. This work presents ExAID (Explainable AI for Dermatology), a novel framework for biomedical image analysis, providing multi-modal concept-based explanations consisting of easy-to-understand textual explanations supplemented by visual maps justifying the predictions. ExAID relies on Concept Activation Vectors to map human concepts to those learnt by arbitrary Deep Learning models in latent space, and Concept Localization Maps to highlight concepts in the input space. This identification of relevant concepts is then used to construct fine-grained textual explanations supplemented by concept-wise location information to provide comprehensive and coherent multi-modal explanations. All information is comprehensively presented in a diagnostic interface for use in clinical routines. An educational mode provides dataset-level explanation statistics and tools for data and model exploration to aid medical research and education. Through rigorous quantitative and qualitative evaluation of ExAID, we show the utility of multi-modal explanations for CAD-assisted scenarios even in case of wrong predictions. We believe that ExAID will provide dermatologists an effective screening tool that they both understand and trust. Moreover, it will be the basis for similar applications in other biomedical imaging fields.
Evaluating Privacy-Preserving Machine Learning in Critical Infrastructures: A Case Study on Time-Series Classification
Nov 29, 2021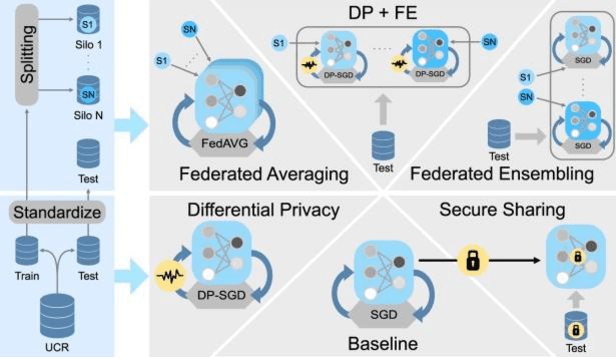
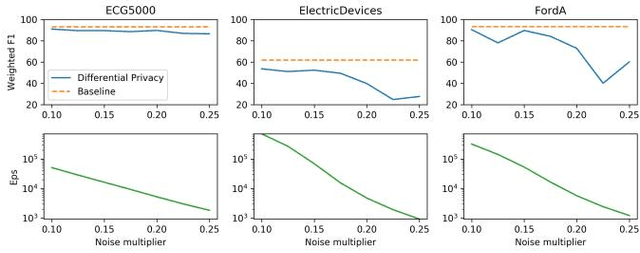
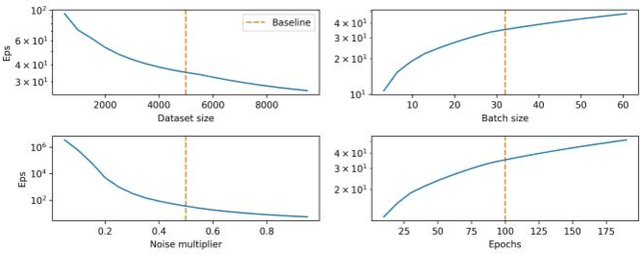
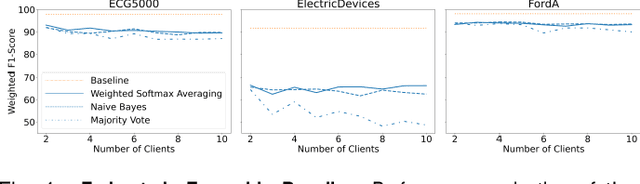
Abstract:With the advent of machine learning in applications of critical infrastructure such as healthcare and energy, privacy is a growing concern in the minds of stakeholders. It is pivotal to ensure that neither the model nor the data can be used to extract sensitive information used by attackers against individuals or to harm whole societies through the exploitation of critical infrastructure. The applicability of machine learning in these domains is mostly limited due to a lack of trust regarding the transparency and the privacy constraints. Various safety-critical use cases (mostly relying on time-series data) are currently underrepresented in privacy-related considerations. By evaluating several privacy-preserving methods regarding their applicability on time-series data, we validated the inefficacy of encryption for deep learning, the strong dataset dependence of differential privacy, and the broad applicability of federated methods.
XAI Handbook: Towards a Unified Framework for Explainable AI
May 14, 2021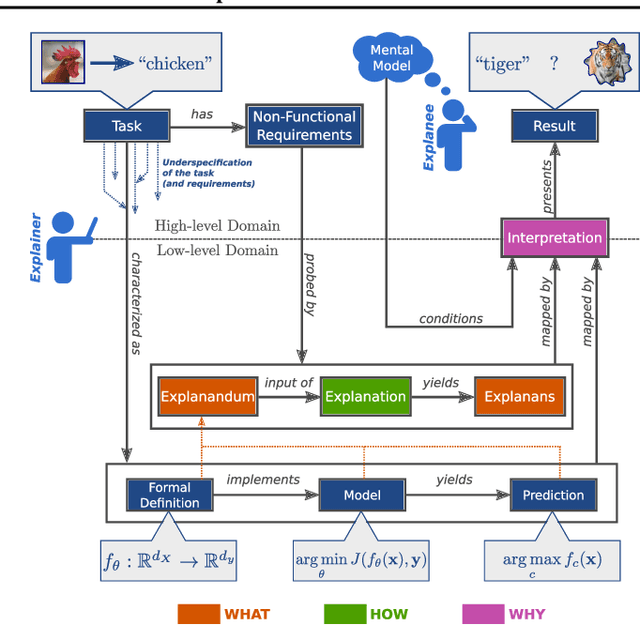
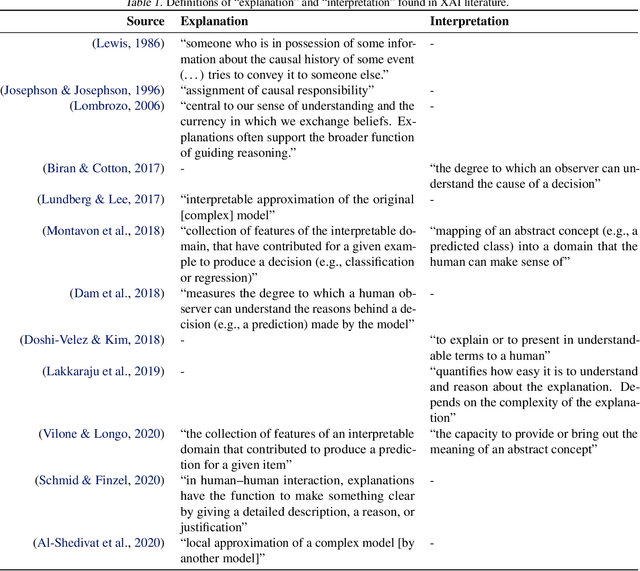

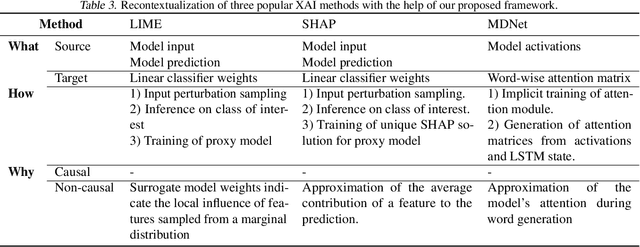
Abstract:The field of explainable AI (XAI) has quickly become a thriving and prolific community. However, a silent, recurrent and acknowledged issue in this area is the lack of consensus regarding its terminology. In particular, each new contribution seems to rely on its own (and often intuitive) version of terms like "explanation" and "interpretation". Such disarray encumbers the consolidation of advances in the field towards the fulfillment of scientific and regulatory demands e.g., when comparing methods or establishing their compliance with respect to biases and fairness constraints. We propose a theoretical framework that not only provides concrete definitions for these terms, but it also outlines all steps necessary to produce explanations and interpretations. The framework also allows for existing contributions to be re-contextualized such that their scope can be measured, thus making them comparable to other methods. We show that this framework is compliant with desiderata on explanations, on interpretability and on evaluation metrics. We present a use-case showing how the framework can be used to compare LIME, SHAP and MDNet, establishing their advantages and shortcomings. Finally, we discuss relevant trends in XAI as well as recommendations for future work, all from the standpoint of our framework.
Deep Learning Based Decision Support for Medicine -- A Case Study on Skin Cancer Diagnosis
Mar 02, 2021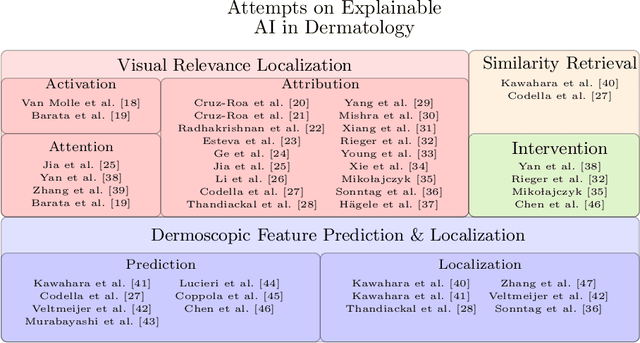
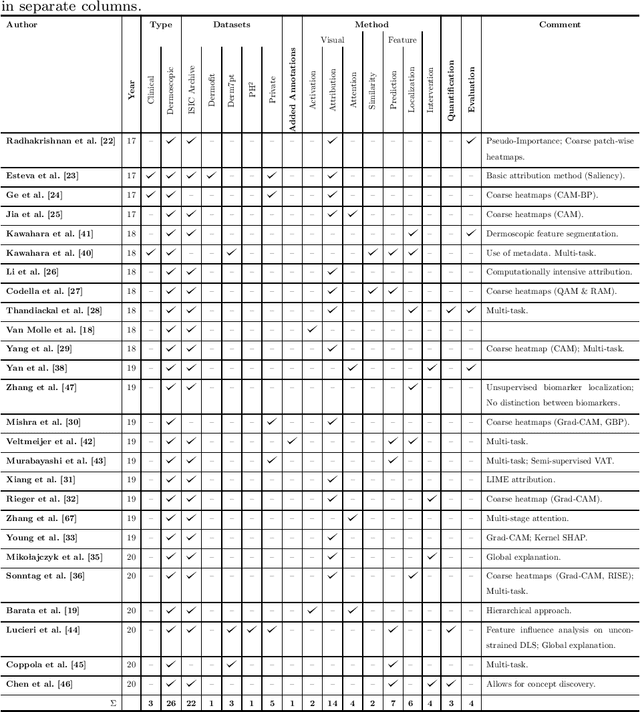
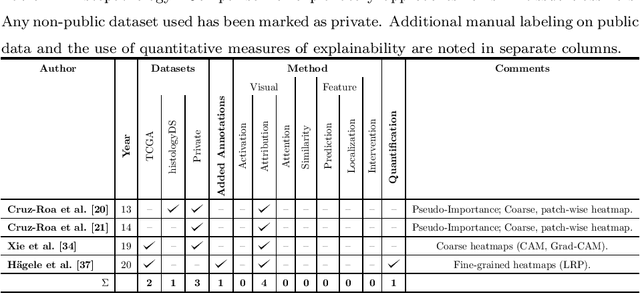
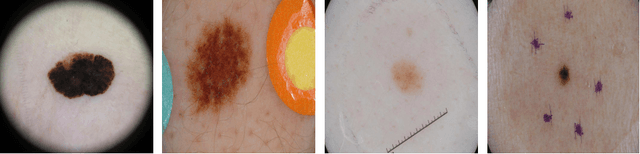
Abstract:Early detection of skin cancers like melanoma is crucial to ensure high chances of survival for patients. Clinical application of Deep Learning (DL)-based Decision Support Systems (DSS) for skin cancer screening has the potential to improve the quality of patient care. The majority of work in the medical AI community focuses on a diagnosis setting that is mainly relevant for autonomous operation. Practical decision support should, however, go beyond plain diagnosis and provide explanations. This paper provides an overview of works towards explainable, DL-based decision support in medical applications with the example of skin cancer diagnosis from clinical, dermoscopic and histopathologic images. Analysis reveals that comparably little attention is payed to the explanation of histopathologic skin images and that current work is dominated by visual relevance maps as well as dermoscopic feature identification. We conclude that future work should focus on meeting the stakeholder's cognitive concepts, providing exhaustive explanations that combine global and local approaches and leverage diverse modalities. Moreover, the possibility to intervene and guide models in case of misbehaviour is identified as a major step towards successful deployment of AI as DL-based DSS and beyond.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge