Victor Volovici
A roadmap to fair and trustworthy prediction model validation in healthcare
Apr 07, 2023Abstract:A prediction model is most useful if it generalizes beyond the development data with external validations, but to what extent should it generalize remains unclear. In practice, prediction models are externally validated using data from very different settings, including populations from other health systems or countries, with predictably poor results. This may not be a fair reflection of the performance of the model which was designed for a specific target population or setting, and may be stretching the expected model generalizability. To address this, we suggest to externally validate a model using new data from the target population to ensure clear implications of validation performance on model reliability, whereas model generalizability to broader settings should be carefully investigated during model development instead of explored post-hoc. Based on this perspective, we propose a roadmap that facilitates the development and application of reliable, fair, and trustworthy artificial intelligence prediction models.
Handling missing values in healthcare data: A systematic review of deep learning-based imputation techniques
Oct 15, 2022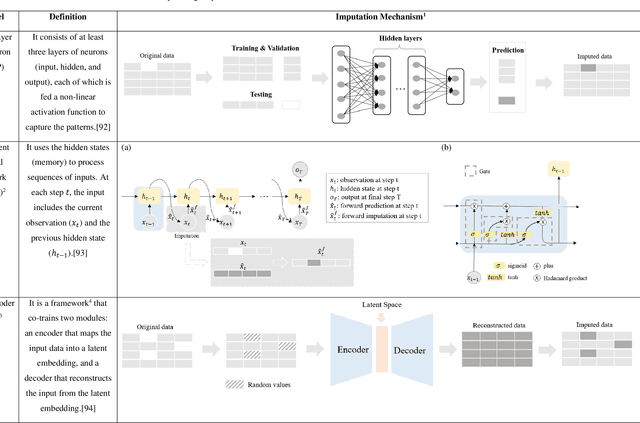
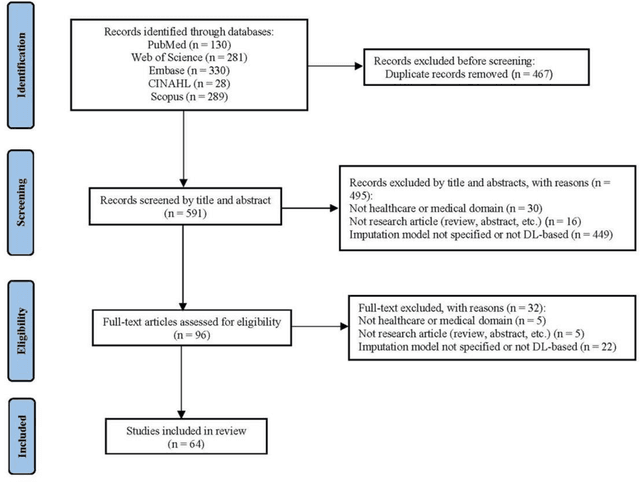
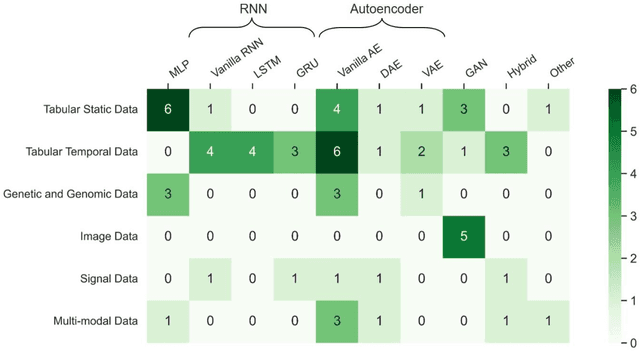
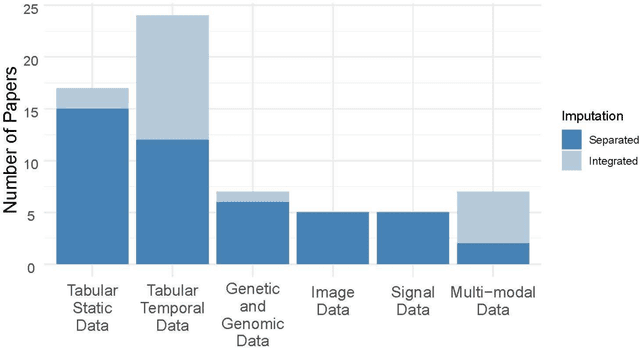
Abstract:Objective: The proper handling of missing values is critical to delivering reliable estimates and decisions, especially in high-stakes fields such as clinical research. The increasing diversity and complexity of data have led many researchers to develop deep learning (DL)-based imputation techniques. We conducted a systematic review to evaluate the use of these techniques, with a particular focus on data types, aiming to assist healthcare researchers from various disciplines in dealing with missing values. Methods: We searched five databases (MEDLINE, Web of Science, Embase, CINAHL, and Scopus) for articles published prior to August 2021 that applied DL-based models to imputation. We assessed selected publications from four perspectives: health data types, model backbone (i.e., main architecture), imputation strategies, and comparison with non-DL-based methods. Based on data types, we created an evidence map to illustrate the adoption of DL models. Results: We included 64 articles, of which tabular static (26.6%, 17/64) and temporal data (37.5%, 24/64) were the most frequently investigated. We found that model backbone(s) differed among data types as well as the imputation strategy. The "integrated" strategy, that is, the imputation task being solved concurrently with downstream tasks, was popular for tabular temporal (50%, 12/24) and multi-modal data (71.4%, 5/7), but limited for other data types. Moreover, DL-based imputation methods yielded better imputation accuracy in most studies, compared with non-DL-based methods. Conclusion: DL-based imputation models can be customized based on data type, addressing the corresponding missing patterns, and its associated "integrated" strategy can enhance the efficacy of imputation, especially in scenarios where data is complex. Future research may focus on the portability and fairness of DL-based models for healthcare data imputation.
AutoScore-Ordinal: An interpretable machine learning framework for generating scoring models for ordinal outcomes
Feb 17, 2022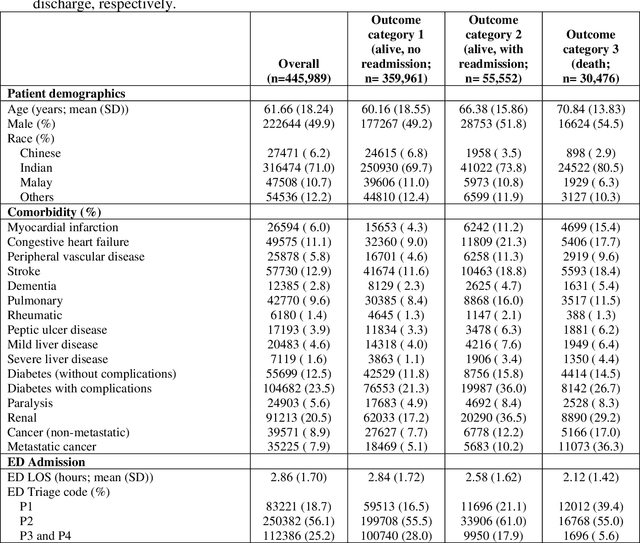
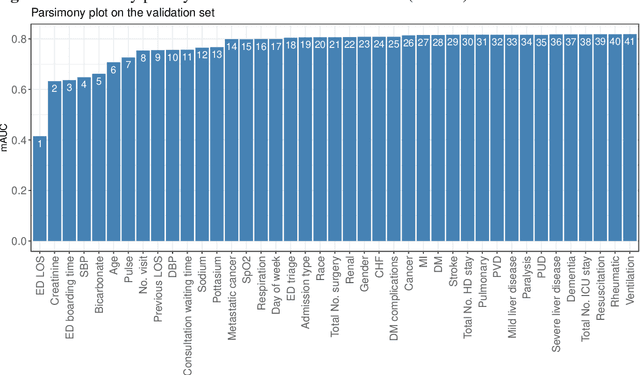
Abstract:Background: Risk prediction models are useful tools in clinical decision-making which help with risk stratification and resource allocations and may lead to a better health care for patients. AutoScore is a machine learning-based automatic clinical score generator for binary outcomes. This study aims to expand the AutoScore framework to provide a tool for interpretable risk prediction for ordinal outcomes. Methods: The AutoScore-Ordinal framework is generated using the same 6 modules of the original AutoScore algorithm including variable ranking, variable transformation, score derivation (from proportional odds models), model selection, score fine-tuning, and model evaluation. To illustrate the AutoScore-Ordinal performance, the method was conducted on electronic health records data from the emergency department at Singapore General Hospital over 2008 to 2017. The model was trained on 70% of the data, validated on 10% and tested on the remaining 20%. Results: This study included 445,989 inpatient cases, where the distribution of the ordinal outcome was 80.7% alive without 30-day readmission, 12.5% alive with 30-day readmission, and 6.8% died inpatient or by day 30 post discharge. Two point-based risk prediction models were developed using two sets of 8 predictor variables identified by the flexible variable selection procedure. The two models indicated reasonably good performance measured by mean area under the receiver operating characteristic curve (0.785 and 0.793) and generalized c-index (0.737 and 0.760), which were comparable to alternative models. Conclusion: AutoScore-Ordinal provides an automated and easy-to-use framework for development and validation of risk prediction models for ordinal outcomes, which can systematically identify potential predictors from high-dimensional data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge