Robyn Fong
Almanac Copilot: Towards Autonomous Electronic Health Record Navigation
May 14, 2024



Abstract:Clinicians spend large amounts of time on clinical documentation, and inefficiencies impact quality of care and increase clinician burnout. Despite the promise of electronic medical records (EMR), the transition from paper-based records has been negatively associated with clinician wellness, in part due to poor user experience, increased burden of documentation, and alert fatigue. In this study, we present Almanac Copilot, an autonomous agent capable of assisting clinicians with EMR-specific tasks such as information retrieval and order placement. On EHR-QA, a synthetic evaluation dataset of 300 common EHR queries based on real patient data, Almanac Copilot obtains a successful task completion rate of 74% (n = 221 tasks) with a mean score of 2.45 over 3 (95% CI:2.34-2.56). By automating routine tasks and streamlining the documentation process, our findings highlight the significant potential of autonomous agents to mitigate the cognitive load imposed on clinicians by current EMR systems.
A Generalizable Deep Learning System for Cardiac MRI
Dec 01, 2023



Abstract:Cardiac MRI allows for a comprehensive assessment of myocardial structure, function, and tissue characteristics. Here we describe a foundational vision system for cardiac MRI, capable of representing the breadth of human cardiovascular disease and health. Our deep learning model is trained via self-supervised contrastive learning, by which visual concepts in cine-sequence cardiac MRI scans are learned from the raw text of the accompanying radiology reports. We train and evaluate our model on data from four large academic clinical institutions in the United States. We additionally showcase the performance of our models on the UK BioBank, and two additional publicly available external datasets. We explore emergent zero-shot capabilities of our system, and demonstrate remarkable performance across a range of tasks; including the problem of left ventricular ejection fraction regression, and the diagnosis of 35 different conditions such as cardiac amyloidosis and hypertrophic cardiomyopathy. We show that our deep learning system is capable of not only understanding the staggering complexity of human cardiovascular disease, but can be directed towards clinical problems of interest yielding impressive, clinical grade diagnostic accuracy with a fraction of the training data typically required for such tasks.
Echocardiogram Foundation Model -- Application 1: Estimating Ejection Fraction
Nov 21, 2023



Abstract:Cardiovascular diseases stand as the primary global cause of mortality. Among the various imaging techniques available for visualising the heart and evaluating its function, echocardiograms emerge as the preferred choice due to their safety and low cost. Quantifying cardiac function based on echocardiograms is very laborious, time-consuming and subject to high interoperator variability. In this work, we introduce EchoAI, an echocardiogram foundation model, that is trained using self-supervised learning (SSL) on 1.5 million echocardiograms. We evaluate our approach by fine-tuning EchoAI to estimate the ejection fraction achieving a mean absolute percentage error of 9.40%. This level of accuracy aligns with the performance of expert sonographers.
Simulating time to event prediction with spatiotemporal echocardiography deep learning
Mar 03, 2021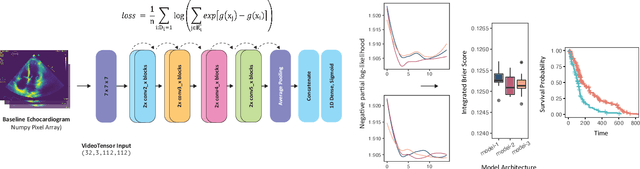
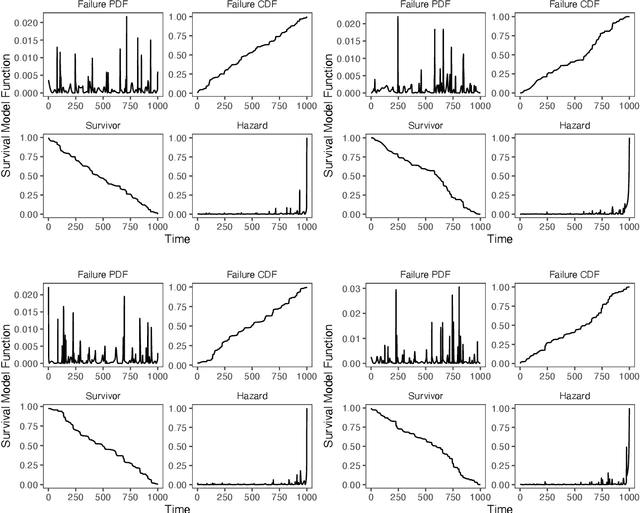
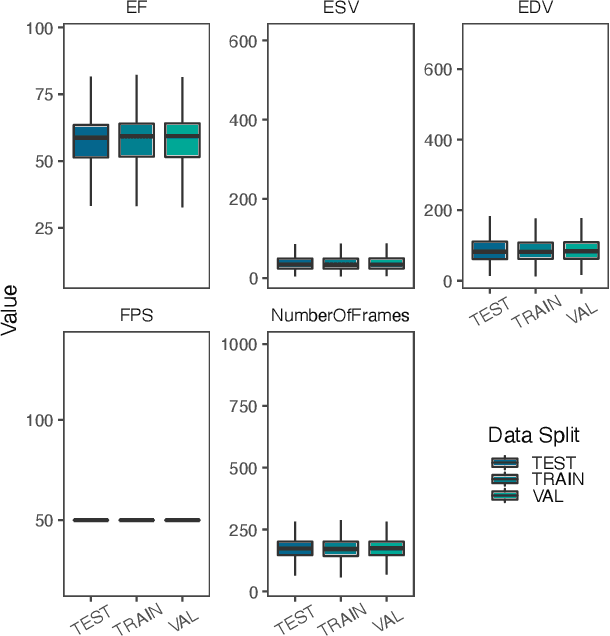
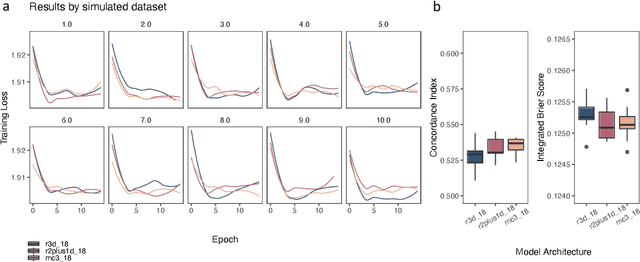
Abstract:Integrating methods for time-to-event prediction with diagnostic imaging modalities is of considerable interest, as accurate estimates of survival requires accounting for censoring of individuals within the observation period. New methods for time-to-event prediction have been developed by extending the cox-proportional hazards model with neural networks. In this paper, to explore the feasibility of these methods when applied to deep learning with echocardiography videos, we utilize the Stanford EchoNet-Dynamic dataset with over 10,000 echocardiograms, and generate simulated survival datasets based on the expert annotated ejection fraction readings. By training on just the simulated survival outcomes, we show that spatiotemporal convolutional neural networks yield accurate survival estimates.
Predicting post-operative right ventricular failure using video-based deep learning
Feb 28, 2021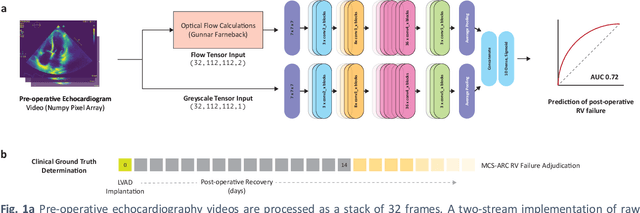
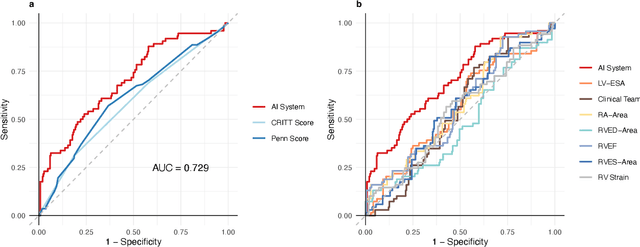
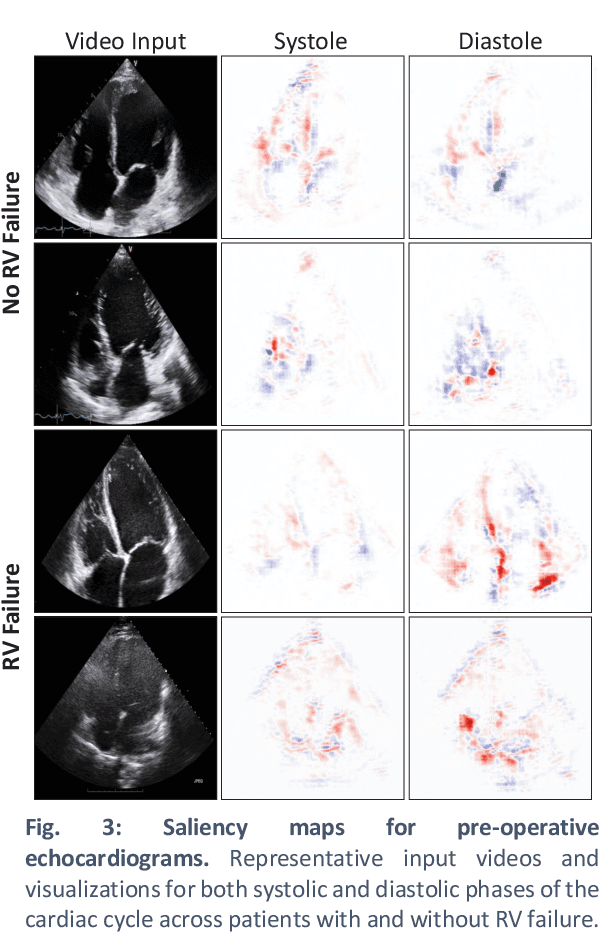
Abstract:Non-invasive and cost effective in nature, the echocardiogram allows for a comprehensive assessment of the cardiac musculature and valves. Despite progressive improvements over the decades, the rich temporally resolved data in echocardiography videos remain underutilized. Human reads of echocardiograms reduce the complex patterns of cardiac wall motion, to a small list of measurements of heart function. Furthermore, all modern echocardiography artificial intelligence (AI) systems are similarly limited by design - automating measurements of the same reductionist metrics rather than utilizing the wealth of data embedded within each echo study. This underutilization is most evident in situations where clinical decision making is guided by subjective assessments of disease acuity, and tools that predict disease onset within clinically actionable timeframes are unavailable. Predicting the likelihood of developing post-operative right ventricular failure (RV failure) in the setting of mechanical circulatory support is one such clinical example. To address this, we developed a novel video AI system trained to predict post-operative right ventricular failure (RV failure), using the full spatiotemporal density of information from pre-operative echocardiography scans. We achieve an AUC of 0.729, specificity of 52% at 80% sensitivity and 46% sensitivity at 80% specificity. Furthermore, we show that our ML system significantly outperforms a team of human experts tasked with predicting RV failure on independent clinical evaluation. Finally, the methods we describe are generalizable to any cardiac clinical decision support application where treatment or patient selection is guided by qualitative echocardiography assessments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge