Nicolas Quach
Simulating time to event prediction with spatiotemporal echocardiography deep learning
Mar 03, 2021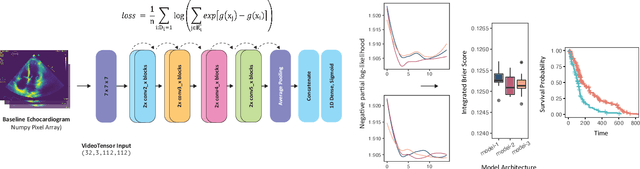
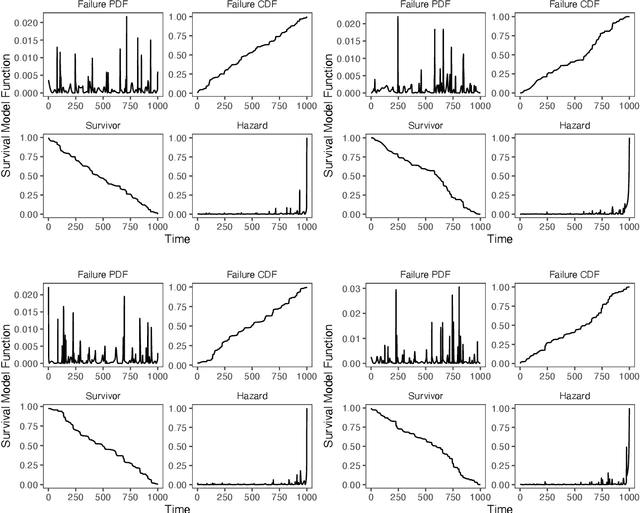
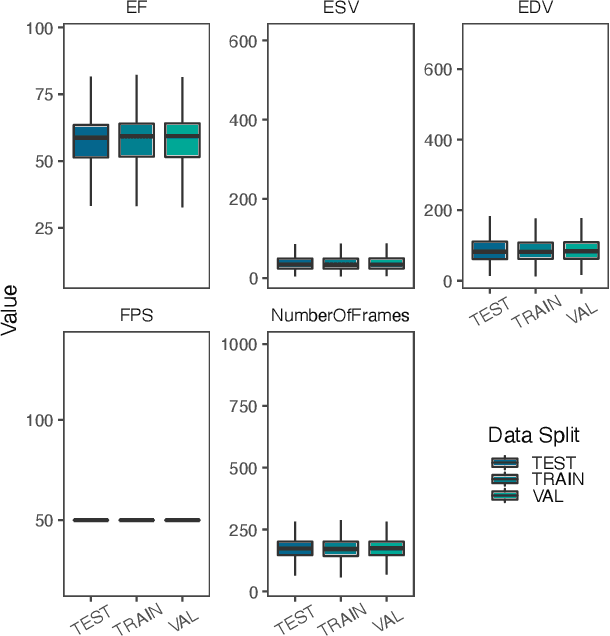
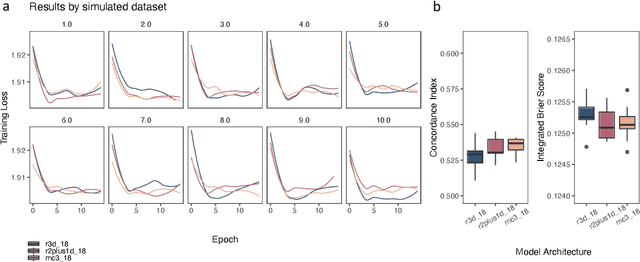
Abstract:Integrating methods for time-to-event prediction with diagnostic imaging modalities is of considerable interest, as accurate estimates of survival requires accounting for censoring of individuals within the observation period. New methods for time-to-event prediction have been developed by extending the cox-proportional hazards model with neural networks. In this paper, to explore the feasibility of these methods when applied to deep learning with echocardiography videos, we utilize the Stanford EchoNet-Dynamic dataset with over 10,000 echocardiograms, and generate simulated survival datasets based on the expert annotated ejection fraction readings. By training on just the simulated survival outcomes, we show that spatiotemporal convolutional neural networks yield accurate survival estimates.
Predicting post-operative right ventricular failure using video-based deep learning
Feb 28, 2021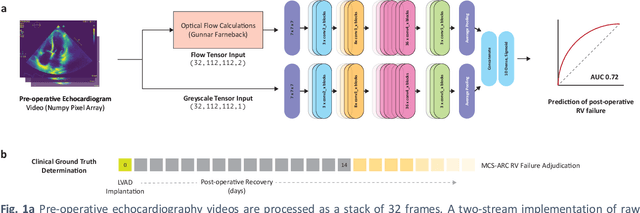
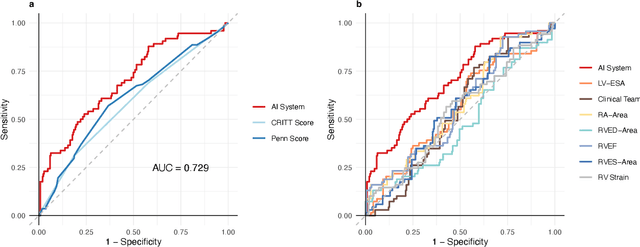
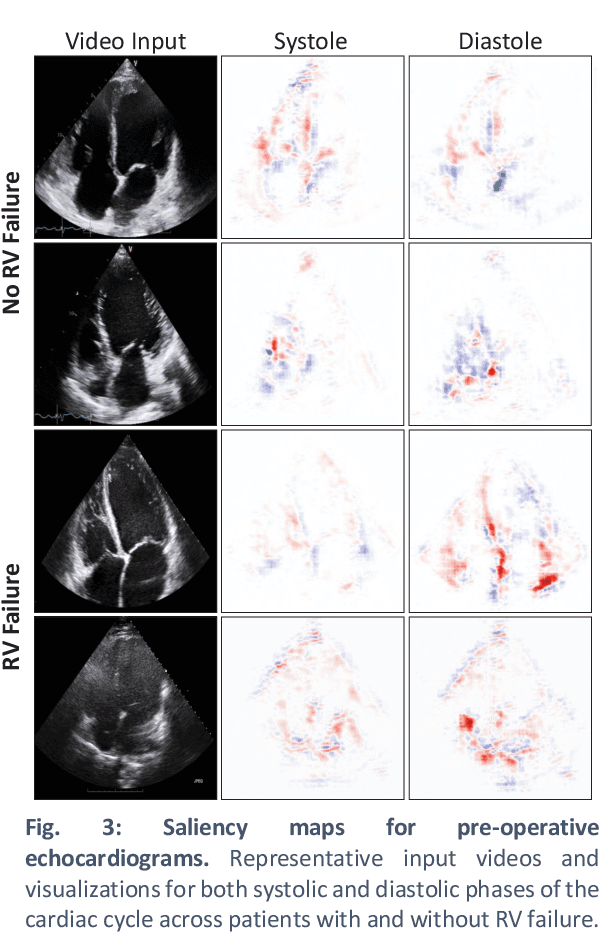
Abstract:Non-invasive and cost effective in nature, the echocardiogram allows for a comprehensive assessment of the cardiac musculature and valves. Despite progressive improvements over the decades, the rich temporally resolved data in echocardiography videos remain underutilized. Human reads of echocardiograms reduce the complex patterns of cardiac wall motion, to a small list of measurements of heart function. Furthermore, all modern echocardiography artificial intelligence (AI) systems are similarly limited by design - automating measurements of the same reductionist metrics rather than utilizing the wealth of data embedded within each echo study. This underutilization is most evident in situations where clinical decision making is guided by subjective assessments of disease acuity, and tools that predict disease onset within clinically actionable timeframes are unavailable. Predicting the likelihood of developing post-operative right ventricular failure (RV failure) in the setting of mechanical circulatory support is one such clinical example. To address this, we developed a novel video AI system trained to predict post-operative right ventricular failure (RV failure), using the full spatiotemporal density of information from pre-operative echocardiography scans. We achieve an AUC of 0.729, specificity of 52% at 80% sensitivity and 46% sensitivity at 80% specificity. Furthermore, we show that our ML system significantly outperforms a team of human experts tasked with predicting RV failure on independent clinical evaluation. Finally, the methods we describe are generalizable to any cardiac clinical decision support application where treatment or patient selection is guided by qualitative echocardiography assessments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge