Philippe Ryvlin
SzCORE: A Seizure Community Open-source Research Evaluation framework for the validation of EEG-based automated seizure detection algorithms
Feb 23, 2024



Abstract:The need for high-quality automated seizure detection algorithms based on electroencephalography (EEG) becomes ever more pressing with the increasing use of ambulatory and long-term EEG monitoring. Heterogeneity in validation methods of these algorithms influences the reported results and makes comprehensive evaluation and comparison challenging. This heterogeneity concerns in particular the choice of datasets, evaluation methodologies, and performance metrics. In this paper, we propose a unified framework designed to establish standardization in the validation of EEG-based seizure detection algorithms. Based on existing guidelines and recommendations, the framework introduces a set of recommendations and standards related to datasets, file formats, EEG data input content, seizure annotation input and output, cross-validation strategies, and performance metrics. We also propose the 10-20 seizure detection benchmark, a machine-learning benchmark based on public datasets converted to a standardized format. This benchmark defines the machine-learning task as well as reporting metrics. We illustrate the use of the benchmark by evaluating a set of existing seizure detection algorithms. The SzCORE (Seizure Community Open-source Research Evaluation) framework and benchmark are made publicly available along with an open-source software library to facilitate research use, while enabling rigorous evaluation of the clinical significance of the algorithms, fostering a collective effort to more optimally detect seizures to improve the lives of people with epilepsy.
EpiDeNet: An Energy-Efficient Approach to Seizure Detection for Embedded Systems
Aug 28, 2023Abstract:Epilepsy is a prevalent neurological disorder that affects millions of individuals globally, and continuous monitoring coupled with automated seizure detection appears as a necessity for effective patient treatment. To enable long-term care in daily-life conditions, comfortable and smart wearable devices with long battery life are required, which in turn set the demand for resource-constrained and energy-efficient computing solutions. In this context, the development of machine learning algorithms for seizure detection faces the challenge of heavily imbalanced datasets. This paper introduces EpiDeNet, a new lightweight seizure detection network, and Sensitivity-Specificity Weighted Cross-Entropy (SSWCE), a new loss function that incorporates sensitivity and specificity, to address the challenge of heavily unbalanced datasets. The proposed EpiDeNet-SSWCE approach demonstrates the successful detection of 91.16% and 92.00% seizure events on two different datasets (CHB-MIT and PEDESITE, respectively), with only four EEG channels. A three-window majority voting-based smoothing scheme combined with the SSWCE loss achieves 3x reduction of false positives to 1.18 FP/h. EpiDeNet is well suited for implementation on low-power embedded platforms, and we evaluate its performance on two ARM Cortex-based platforms (M4F/M7) and two parallel ultra-low power (PULP) systems (GAP8, GAP9). The most efficient implementation (GAP9) achieves an energy efficiency of 40 GMAC/s/W, with an energy consumption per inference of only 0.051 mJ at high performance (726.46 MMAC/s), outperforming the best ARM Cortex-based solutions by approximately 160x in energy efficiency. The EpiDeNet-SSWCE method demonstrates effective and accurate seizure detection performance on heavily imbalanced datasets, while being suited for implementation on energy-constrained platforms.
Towards Long-term Non-invasive Monitoring for Epilepsy via Wearable EEG Devices
Jun 17, 2021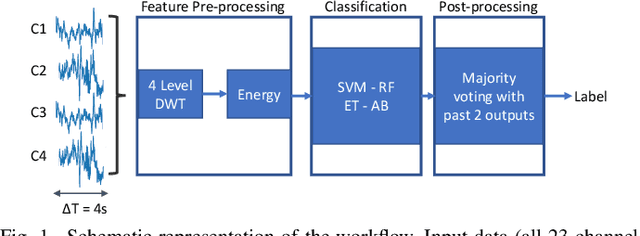

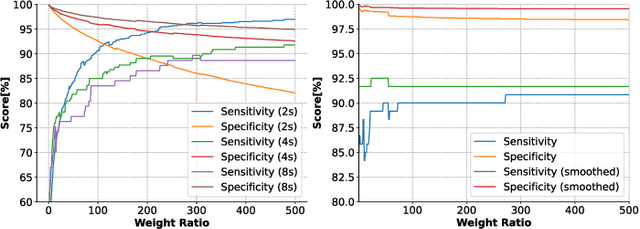

Abstract:We present the implementation of seizure detection algorithms based on a minimal number of EEG channels on a parallel ultra-low-power embedded platform. The analyses are based on the CHB-MIT dataset, and include explorations of different classification approaches (Support Vector Machines, Random Forest, Extra Trees, AdaBoost) and different pre/post-processing techniques to maximize sensitivity while guaranteeing no false alarms. We analyze global and subject-specific approaches, considering all 23-electrodes or only 4 temporal channels. For 8s window size and subject-specific approach, we report zero false positives and 100% sensitivity. These algorithms are parallelized and optimized for a parallel ultra-low power (PULP) platform, enabling 300h of continuous monitoring on a 300 mAh battery, in a wearable form factor and power budget. These results pave the way for the implementation of affordable, wearable, long-term epilepsy monitoring solutions with low false-positive rates and high sensitivity, meeting both patient and caregiver requirements.
Interpreting Deep Learning Models for Epileptic Seizure Detection on EEG signals
Dec 22, 2020



Abstract:While Deep Learning (DL) is often considered the state-of-the art for Artificial Intelligence-based medical decision support, it remains sparsely implemented in clinical practice and poorly trusted by clinicians due to insufficient interpretability of neural network models. We have tackled this issue by developing interpretable DL models in the context of online detection of epileptic seizure, based on EEG signal. This has conditioned the preparation of the input signals, the network architecture, and the post-processing of the output in line with the domain knowledge. Specifically, we focused the discussion on three main aspects: 1) how to aggregate the classification results on signal segments provided by the DL model into a larger time scale, at the seizure-level; 2) what are the relevant frequency patterns learned in the first convolutional layer of different models, and their relation with the delta, theta, alpha, beta and gamma frequency bands on which the visual interpretation of EEG is based; and 3) the identification of the signal waveforms with larger contribution towards the ictal class, according to the activation differences highlighted using the DeepLIFT method. Results show that the kernel size in the first layer determines the interpretability of the extracted features and the sensitivity of the trained models, even though the final performance is very similar after post-processing. Also, we found that amplitude is the main feature leading to an ictal prediction, suggesting that a larger patient population would be required to learn more complex frequency patterns. Still, our methodology was successfully able to generalize patient inter-variability for the majority of the studied population with a classification F1-score of 0.873 and detecting 90% of the seizures.
Synthetic Epileptic Brain Activities Using Generative Adversarial Networks
Jul 22, 2019



Abstract:Epilepsy is a chronic neurological disorder affecting more than 65 million people worldwide and manifested by recurrent unprovoked seizures. The unpredictability of seizures not only degrades the quality of life of the patients, but it can also be life-threatening. Modern systems monitoring electroencephalography (EEG) signals are being currently developed with the view to detect epileptic seizures in order to alert caregivers and reduce the impact of seizures on patients' quality of life. Such seizure detection systems employ state-of-the-art machine learning algorithms that require a considerably large amount of labeled personal data for training. However, acquiring EEG signals of epileptic seizures is a costly and time-consuming process for medical experts and patients, currently requiring in-hospital recordings in specialized units. In this work, we generate synthetic seizure-like brain electrical activities, i.e., EEG signals, that can be used to train seizure detection algorithms, alleviating the need for recorded data. First, we train a Generative Adversarial Network (GAN) with data from 30 epilepsy patients. Then, we generate synthetic personalized training sets for new, unseen patients, which overall yield higher detection performance than the real-data training sets. We demonstrate our results using the datasets from the EPILEPSIAE Project, one of the world's largest public databases for seizure detection.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge