Andrea Cossettini
Wearable and Ultra-Low-Power Fusion of EMG and A-Mode US for Hand-Wrist Kinematic Tracking
Oct 02, 2025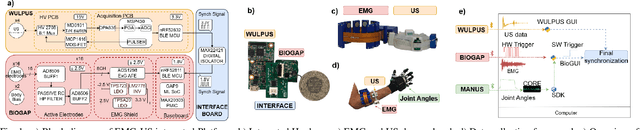
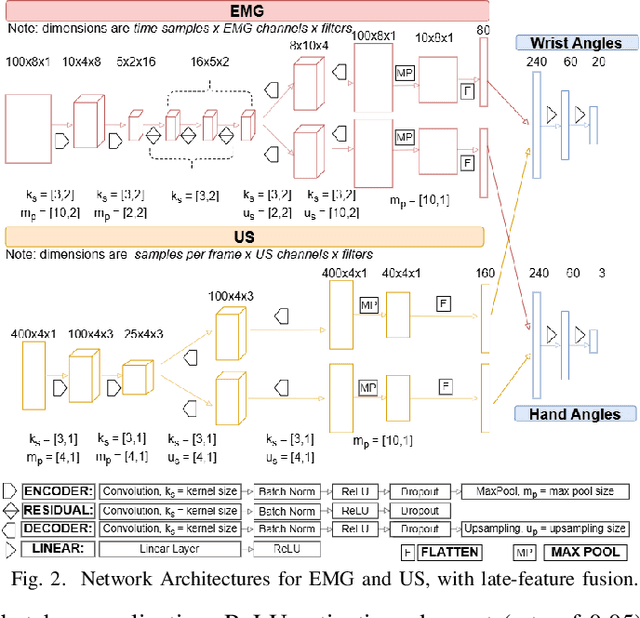
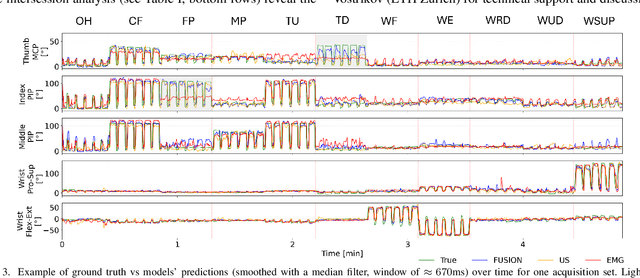
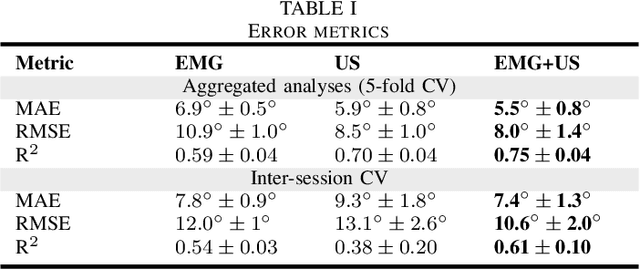
Abstract:Hand gesture recognition based on biosignals has shown strong potential for developing intuitive human-machine interaction strategies that closely mimic natural human behavior. In particular, sensor fusion approaches have gained attention for combining complementary information and overcoming the limitations of individual sensing modalities, thereby enabling more robust and reliable systems. Among them, the fusion of surface electromyography (EMG) and A-mode ultrasound (US) is very promising. However, prior solutions rely on power-hungry platforms unsuitable for multi-day use and are limited to discrete gesture classification. In this work, we present an ultra-low-power (sub-50 mW) system for concurrent acquisition of 8-channel EMG and 4-channel A-mode US signals, integrating two state-of-the-art platforms into fully wearable, dry-contact armbands. We propose a framework for continuous tracking of 23 degrees of freedom (DoFs), 20 for the hand and 3 for the wrist, using a kinematic glove for ground-truth labeling. Our method employs lightweight encoder-decoder architectures with multi-task learning to simultaneously estimate hand and wrist joint angles. Experimental results under realistic sensor repositioning conditions demonstrate that EMG-US fusion achieves a root mean squared error of $10.6^\circ\pm2.0^\circ$, compared to $12.0^\circ\pm1^\circ$ for EMG and $13.1^\circ\pm2.6^\circ$ for US, and a R$^2$ score of $0.61\pm0.1$, with $0.54\pm0.03$ for EMG and $0.38\pm0.20$ for US.
A Parallel Ultra-Low Power Silent Speech Interface based on a Wearable, Fully-dry EMG Neckband
Sep 26, 2025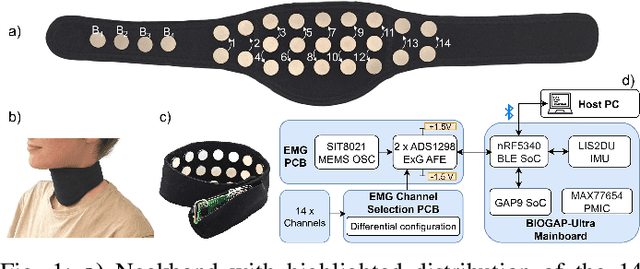
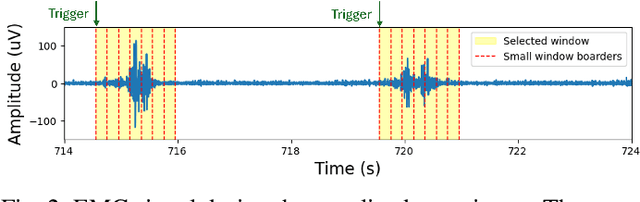
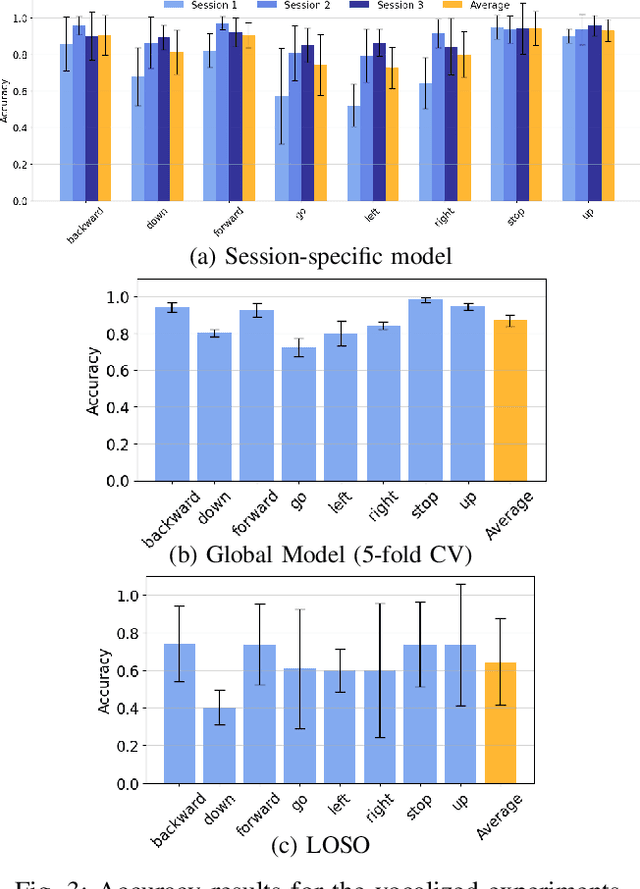
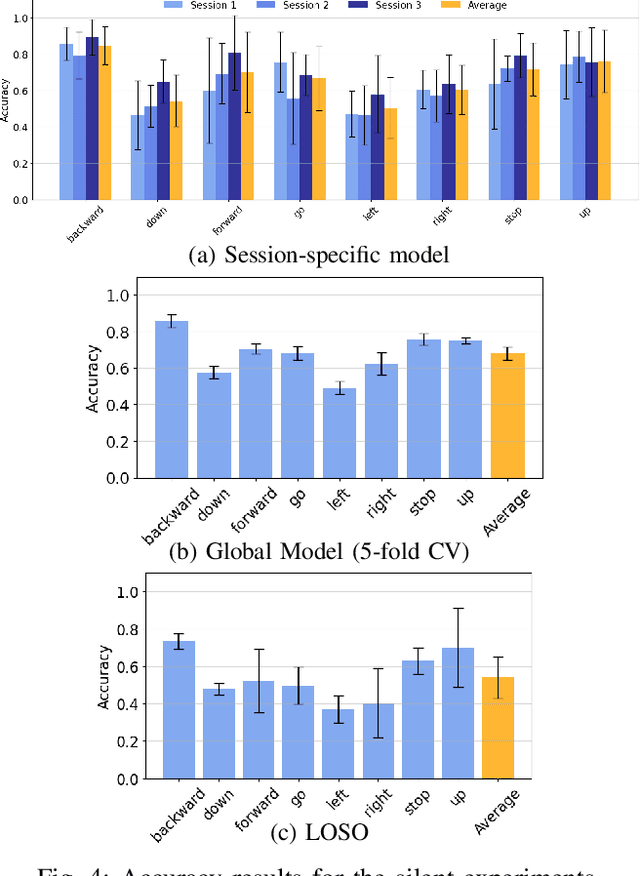
Abstract:We present a wearable, fully-dry, and ultra-low power EMG system for silent speech recognition, integrated into a textile neckband to enable comfortable, non-intrusive use. The system features 14 fully-differential EMG channels and is based on the BioGAP-Ultra platform for ultra-low power (22 mW) biosignal acquisition and wireless transmission. We evaluate its performance on eight speech commands under both vocalized and silent articulation, achieving average classification accuracies of 87$\pm$3% and 68$\pm$3% respectively, with a 5-fold CV approach. To mimic everyday-life conditions, we introduce session-to-session variability by repositioning the neckband between sessions, achieving leave-one-session-out accuracies of 64$\pm$18% and 54$\pm$7% for the vocalized and silent experiments, respectively. These results highlight the robustness of the proposed approach and the promise of energy-efficient silent-speech decoding.
ListenToJESD204B: A Lightweight Open-Source JESD204B IP Core for FPGA-Based Ultrasound Acquisition systems
Aug 20, 2025Abstract:The demand for hundreds of tightly synchronized channels operating at tens of MSPS in ultrasound systems exceeds conventional low-voltage differential signaling links' bandwidth, pin count, and latency. Although the JESD204B serial interface mitigates these limitations, commercial FPGA IP cores are proprietary, costly, and resource-intensive. We present ListenToJESD204B, an open-source receiver IP core released under a permissive Solderpad 0.51 license for AMD Xilinx Zynq UltraScale+ devices. Written in synthesizable SystemVerilog, the core supports four GTH/GTY lanes at 12.8 Gb/s and provides cycle-accurate AXI-Stream data alongside deterministic Subclass~1 latency. It occupies only 107 configurable logic blocks (approximately 437 LUTs), representing a 79\% reduction compared to comparable commercially available IP. A modular data path featuring per-lane elastic buffers, SYSREF-locked LMFC generation, and optional LFSR descrambling facilitates scaling to high lane counts. We verified protocol compliance through simulation against the Xilinx JESD204C IP in JESD204B mode and on hardware using TI AFE58JD48 ADCs. Block stability was verified by streaming 80 MSPS, 16-bit samples over two 12.8 Gb/s links for 30 minutes with no errors.
Real-Time, Single-Ear, Wearable ECG Reconstruction, R-Peak Detection, and HR/HRV Monitoring
May 03, 2025Abstract:Biosignal monitoring, in particular heart activity through heart rate (HR) and heart rate variability (HRV) tracking, is vital in enabling continuous, non-invasive tracking of physiological and cognitive states. Recent studies have explored compact, head-worn devices for HR and HRV monitoring to improve usability and reduce stigma. However, this approach is challenged by the current reliance on wet electrodes, which limits usability, the weakness of ear-derived signals, making HR/HRV extraction more complex, and the incompatibility of current algorithms for embedded deployment. This work introduces a single-ear wearable system for real-time ECG (Electrocardiogram) parameter estimation, which directly runs on BioGAP, an energy-efficient device for biosignal acquisition and processing. By combining SoA in-ear electrode technology, an optimized DeepMF algorithm, and BioGAP, our proposed subject-independent approach allows for robust extraction of HR/HRV parameters directly on the device with just 36.7 uJ/inference at comparable performance with respect to the current state-of-the-art architecture, achieving 0.49 bpm and 25.82 ms for HR/HRV mean errors, respectively and an estimated battery life of 36h with a total system power consumption of 7.6 mW. Clinical relevance: The ability to reconstruct ECG signals and extract HR and HRV paves the way for continuous, unobtrusive cardiovascular monitoring with head-worn devices. In particular, the integration of cardiovascular measurements in everyday-use devices (such as earbuds) has potential in continuous at-home monitoring to enable early detection of cardiovascular irregularities.
CEReBrO: Compact Encoder for Representations of Brain Oscillations Using Efficient Alternating Attention
Jan 18, 2025Abstract:Electroencephalograph (EEG) is a crucial tool for studying brain activity. Recently, self-supervised learning methods leveraging large unlabeled datasets have emerged as a potential solution to the scarcity of widely available annotated EEG data. However, current methods suffer from at least one of the following limitations: i) sub-optimal EEG signal modeling, ii) model sizes in the hundreds of millions of trainable parameters, and iii) reliance on private datasets and/or inconsistent public benchmarks, hindering reproducibility. To address these challenges, we introduce a Compact Encoder for Representations of Brain Oscillations using alternating attention (CEReBrO), a new small EEG foundation model. Our tokenization scheme represents EEG signals at a per-channel patch granularity. We propose an alternating attention mechanism that jointly models intra-channel temporal dynamics and inter-channel spatial correlations, achieving 2x speed improvement with 6x less memory required compared to standard self-attention. We present several model sizes ranging from 3.6 million to 85 million parameters. Pre-trained on over 20,000 hours of publicly available scalp EEG recordings with diverse channel configurations, our models set new benchmarks in emotion detection and seizure detection tasks, with competitive performance in anomaly classification and gait prediction. This validates our models' effectiveness and effictiveness.
An Ultra-Low Power Wearable BMI System with Continual Learning Capabilities
Sep 16, 2024



Abstract:Driven by the progress in efficient embedded processing, there is an accelerating trend toward running machine learning models directly on wearable Brain-Machine Interfaces (BMIs) to improve portability and privacy and maximize battery life. However, achieving low latency and high classification performance remains challenging due to the inherent variability of electroencephalographic (EEG) signals across sessions and the limited onboard resources. This work proposes a comprehensive BMI workflow based on a CNN-based Continual Learning (CL) framework, allowing the system to adapt to inter-session changes. The workflow is deployed on a wearable, parallel ultra-low power BMI platform (BioGAP). Our results based on two in-house datasets, Dataset A and Dataset B, show that the CL workflow improves average accuracy by up to 30.36% and 10.17%, respectively. Furthermore, when implementing the continual learning on a Parallel Ultra-Low Power (PULP) microcontroller (GAP9), it achieves an energy consumption as low as 0.45mJ per inference and an adaptation time of only 21.5ms, yielding around 25h of battery life with a small 100mAh, 3.7V battery on BioGAP. Our setup, coupled with the compact CNN model and on-device CL capabilities, meets users' needs for improved privacy, reduced latency, and enhanced inter-session performance, offering good promise for smart embedded real-world BMIs.
Train-On-Request: An On-Device Continual Learning Workflow for Adaptive Real-World Brain Machine Interfaces
Sep 13, 2024



Abstract:Brain-machine interfaces (BMIs) are expanding beyond clinical settings thanks to advances in hardware and algorithms. However, they still face challenges in user-friendliness and signal variability. Classification models need periodic adaptation for real-life use, making an optimal re-training strategy essential to maximize user acceptance and maintain high performance. We propose TOR, a train-on-request workflow that enables user-specific model adaptation to novel conditions, addressing signal variability over time. Using continual learning, TOR preserves knowledge across sessions and mitigates inter-session variability. With TOR, users can refine, on demand, the model through on-device learning (ODL) to enhance accuracy adapting to changing conditions. We evaluate the proposed methodology on a motor-movement dataset recorded with a non-stigmatizing wearable BMI headband, achieving up to 92% accuracy and a re-calibration time as low as 1.6 minutes, a 46% reduction compared to a naive transfer learning workflow. We additionally demonstrate that TOR is suitable for ODL in extreme edge settings by deploying the training procedure on a RISC-V ultra-low-power SoC (GAP9), resulting in 21.6 ms of latency and 1 mJ of energy consumption per training step. To the best of our knowledge, this work is the first demonstration of an online, energy-efficient, dynamic adaptation of a BMI model to the intrinsic variability of EEG signals in real-time settings.
BISeizuRe: BERT-Inspired Seizure Data Representation to Improve Epilepsy Monitoring
Jun 27, 2024



Abstract:This study presents a novel approach for EEG-based seizure detection leveraging a BERT-based model. The model, BENDR, undergoes a two-phase training process. Initially, it is pre-trained on the extensive Temple University Hospital EEG Corpus (TUEG), a 1.5 TB dataset comprising over 10,000 subjects, to extract common EEG data patterns. Subsequently, the model is fine-tuned on the CHB-MIT Scalp EEG Database, consisting of 664 EEG recordings from 24 pediatric patients, of which 198 contain seizure events. Key contributions include optimizing fine-tuning on the CHB-MIT dataset, where the impact of model architecture, pre-processing, and post-processing techniques are thoroughly examined to enhance sensitivity and reduce false positives per hour (FP/h). We also explored custom training strategies to ascertain the most effective setup. The model undergoes a novel second pre-training phase before subject-specific fine-tuning, enhancing its generalization capabilities. The optimized model demonstrates substantial performance enhancements, achieving as low as 0.23 FP/h, 2.5$\times$ lower than the baseline model, with a lower but still acceptable sensitivity rate, showcasing the effectiveness of applying a BERT-based approach on EEG-based seizure detection.
GAPses: Versatile smart glasses for comfortable and fully-dry acquisition and parallel ultra-low-power processing of EEG and EOG
Jun 12, 2024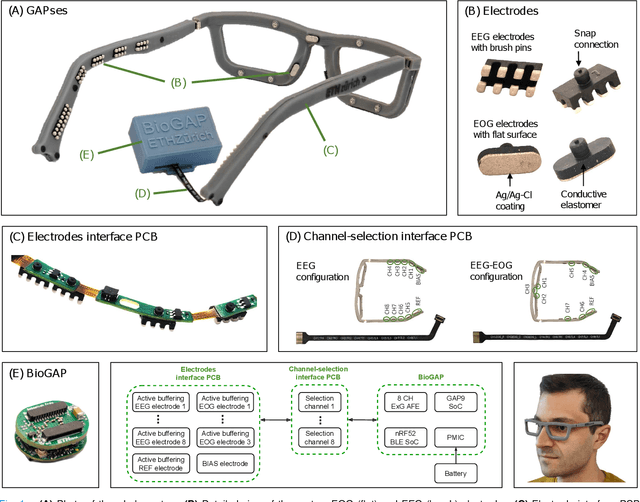
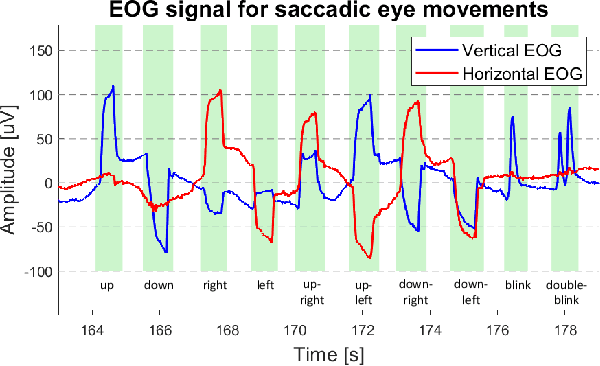
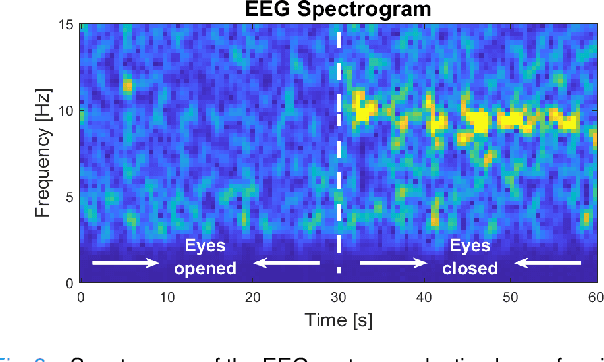
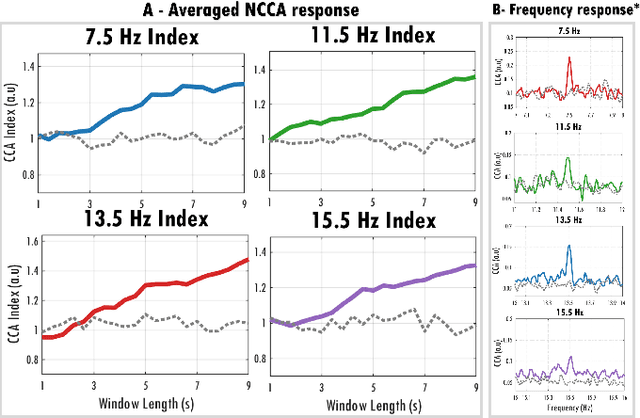
Abstract:Recent advancements in head-mounted wearable technology are revolutionizing the field of biopotential measurement, but the integration of these technologies into practical, user-friendly devices remains challenging due to issues with design intrusiveness, comfort, and data privacy. To address these challenges, this paper presents GAPSES, a novel smart glasses platform designed for unobtrusive, comfortable, and secure acquisition and processing of electroencephalography (EEG) and electrooculography (EOG) signals. We introduce a direct electrode-electronics interface with custom fully dry soft electrodes to enhance comfort for long wear. An integrated parallel ultra-low-power RISC-V processor (GAP9, Greenwaves Technologies) processes data at the edge, thereby eliminating the need for continuous data streaming through a wireless link, enhancing privacy, and increasing system reliability in adverse channel conditions. We demonstrate the broad applicability of the designed prototype through validation in a number of EEG-based interaction tasks, including alpha waves, steady-state visual evoked potential analysis, and motor movement classification. Furthermore, we demonstrate an EEG-based biometric subject recognition task, where we reach a sensitivity and specificity of 98.87% and 99.86% respectively, with only 8 EEG channels and an energy consumption per inference on the edge as low as 121 uJ. Moreover, in an EOG-based eye movement classification task, we reach an accuracy of 96.68% on 11 classes, resulting in an information transfer rate of 94.78 bit/min, which can be further increased to 161.43 bit/min by reducing the accuracy to 81.43%. The deployed implementation has an energy consumption of 24 uJ per inference and a total system power of only 16.28 mW, allowing for continuous operation of more than 12 h with a small 75 mAh battery.
SzCORE: A Seizure Community Open-source Research Evaluation framework for the validation of EEG-based automated seizure detection algorithms
Feb 23, 2024



Abstract:The need for high-quality automated seizure detection algorithms based on electroencephalography (EEG) becomes ever more pressing with the increasing use of ambulatory and long-term EEG monitoring. Heterogeneity in validation methods of these algorithms influences the reported results and makes comprehensive evaluation and comparison challenging. This heterogeneity concerns in particular the choice of datasets, evaluation methodologies, and performance metrics. In this paper, we propose a unified framework designed to establish standardization in the validation of EEG-based seizure detection algorithms. Based on existing guidelines and recommendations, the framework introduces a set of recommendations and standards related to datasets, file formats, EEG data input content, seizure annotation input and output, cross-validation strategies, and performance metrics. We also propose the 10-20 seizure detection benchmark, a machine-learning benchmark based on public datasets converted to a standardized format. This benchmark defines the machine-learning task as well as reporting metrics. We illustrate the use of the benchmark by evaluating a set of existing seizure detection algorithms. The SzCORE (Seizure Community Open-source Research Evaluation) framework and benchmark are made publicly available along with an open-source software library to facilitate research use, while enabling rigorous evaluation of the clinical significance of the algorithms, fostering a collective effort to more optimally detect seizures to improve the lives of people with epilepsy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge