Peixin Dong
Potential of Multimodal Large Language Models for Data Mining of Medical Images and Free-text Reports
Jul 08, 2024



Abstract:Medical images and radiology reports are crucial for diagnosing medical conditions, highlighting the importance of quantitative analysis for clinical decision-making. However, the diversity and cross-source heterogeneity of these data challenge the generalizability of current data-mining methods. Multimodal large language models (MLLMs) have recently transformed many domains, significantly affecting the medical field. Notably, Gemini-Vision-series (Gemini) and GPT-4-series (GPT-4) models have epitomized a paradigm shift in Artificial General Intelligence (AGI) for computer vision, showcasing their potential in the biomedical domain. In this study, we evaluated the performance of the Gemini, GPT-4, and 4 popular large models for an exhaustive evaluation across 14 medical imaging datasets, including 5 medical imaging categories (dermatology, radiology, dentistry, ophthalmology, and endoscopy), and 3 radiology report datasets. The investigated tasks encompass disease classification, lesion segmentation, anatomical localization, disease diagnosis, report generation, and lesion detection. Our experimental results demonstrated that Gemini-series models excelled in report generation and lesion detection but faces challenges in disease classification and anatomical localization. Conversely, GPT-series models exhibited proficiency in lesion segmentation and anatomical localization but encountered difficulties in disease diagnosis and lesion detection. Additionally, both the Gemini series and GPT series contain models that have demonstrated commendable generation efficiency. While both models hold promise in reducing physician workload, alleviating pressure on limited healthcare resources, and fostering collaboration between clinical practitioners and artificial intelligence technologies, substantial enhancements and comprehensive validations remain imperative before clinical deployment.
ChatRadio-Valuer: A Chat Large Language Model for Generalizable Radiology Report Generation Based on Multi-institution and Multi-system Data
Oct 10, 2023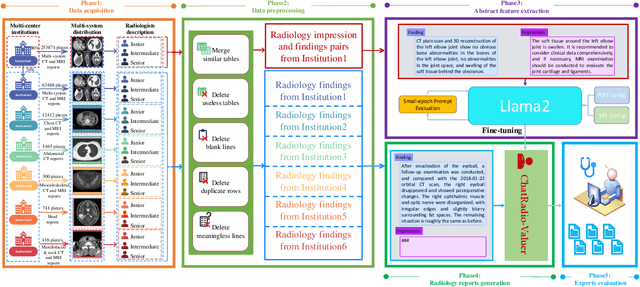
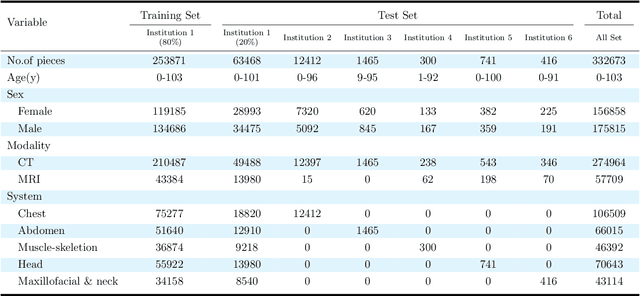
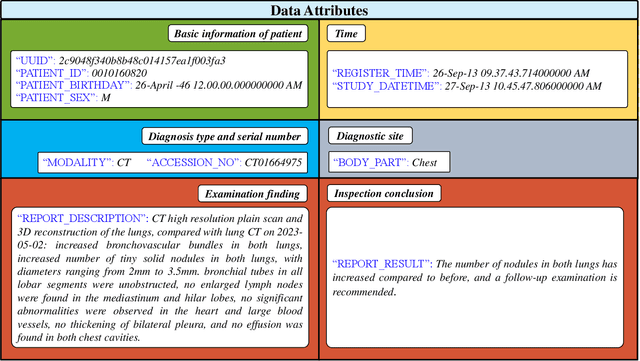
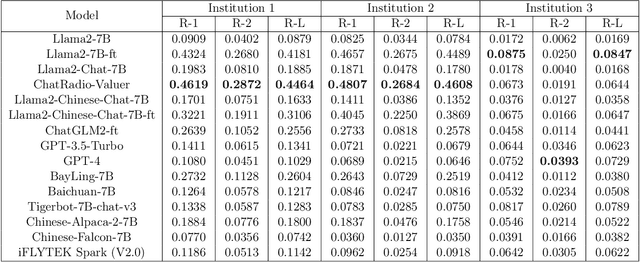
Abstract:Radiology report generation, as a key step in medical image analysis, is critical to the quantitative analysis of clinically informed decision-making levels. However, complex and diverse radiology reports with cross-source heterogeneity pose a huge generalizability challenge to the current methods under massive data volume, mainly because the style and normativity of radiology reports are obviously distinctive among institutions, body regions inspected and radiologists. Recently, the advent of large language models (LLM) offers great potential for recognizing signs of health conditions. To resolve the above problem, we collaborate with the Second Xiangya Hospital in China and propose ChatRadio-Valuer based on the LLM, a tailored model for automatic radiology report generation that learns generalizable representations and provides a basis pattern for model adaptation in sophisticated analysts' cases. Specifically, ChatRadio-Valuer is trained based on the radiology reports from a single institution by means of supervised fine-tuning, and then adapted to disease diagnosis tasks for human multi-system evaluation (i.e., chest, abdomen, muscle-skeleton, head, and maxillofacial $\&$ neck) from six different institutions in clinical-level events. The clinical dataset utilized in this study encompasses a remarkable total of \textbf{332,673} observations. From the comprehensive results on engineering indicators, clinical efficacy and deployment cost metrics, it can be shown that ChatRadio-Valuer consistently outperforms state-of-the-art models, especially ChatGPT (GPT-3.5-Turbo) and GPT-4 et al., in terms of the diseases diagnosis from radiology reports. ChatRadio-Valuer provides an effective avenue to boost model generalization performance and alleviate the annotation workload of experts to enable the promotion of clinical AI applications in radiology reports.
Evaluating Large Language Models for Radiology Natural Language Processing
Jul 27, 2023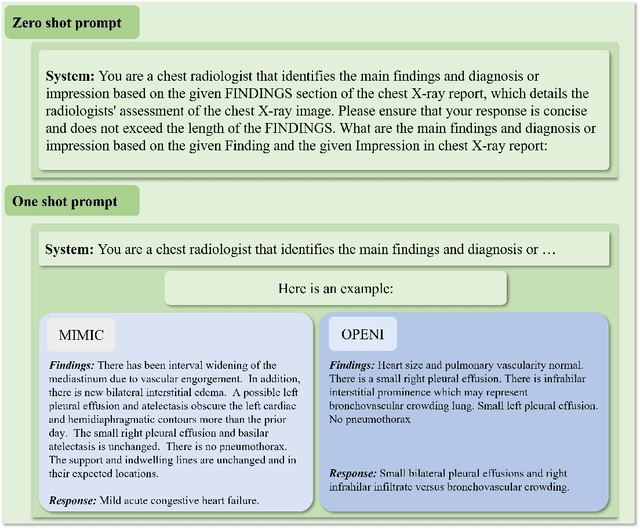
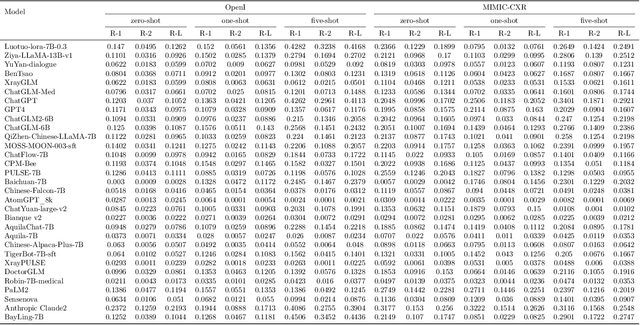
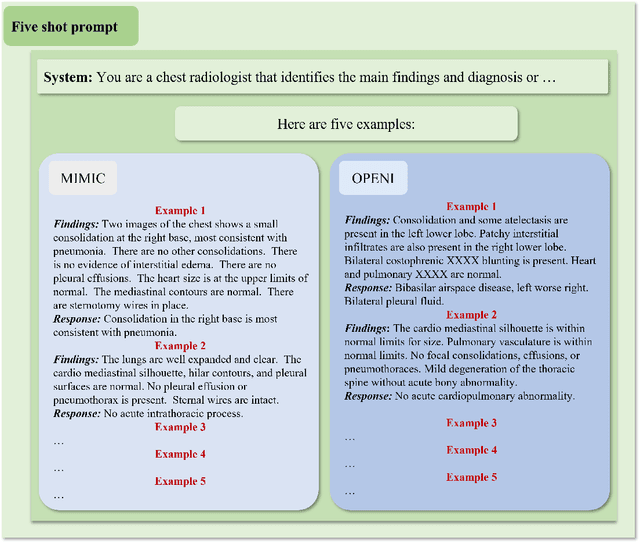
Abstract:The rise of large language models (LLMs) has marked a pivotal shift in the field of natural language processing (NLP). LLMs have revolutionized a multitude of domains, and they have made a significant impact in the medical field. Large language models are now more abundant than ever, and many of these models exhibit bilingual capabilities, proficient in both English and Chinese. However, a comprehensive evaluation of these models remains to be conducted. This lack of assessment is especially apparent within the context of radiology NLP. This study seeks to bridge this gap by critically evaluating thirty two LLMs in interpreting radiology reports, a crucial component of radiology NLP. Specifically, the ability to derive impressions from radiologic findings is assessed. The outcomes of this evaluation provide key insights into the performance, strengths, and weaknesses of these LLMs, informing their practical applications within the medical domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge