Michael Bacci
Reproducible evaluation of classification methods in Alzheimer's disease: framework and application to MRI and PET data
Aug 20, 2018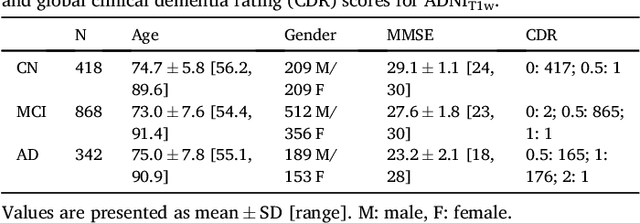
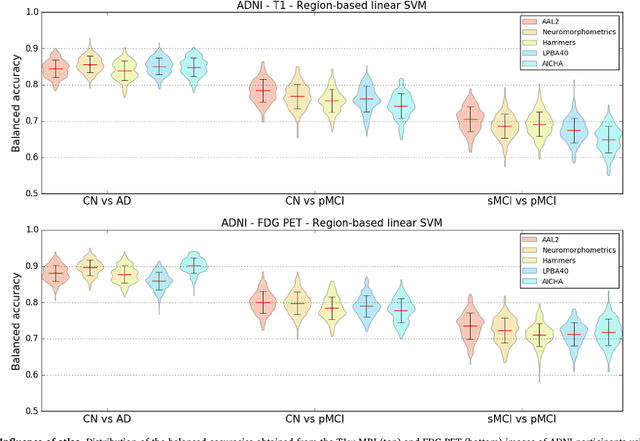
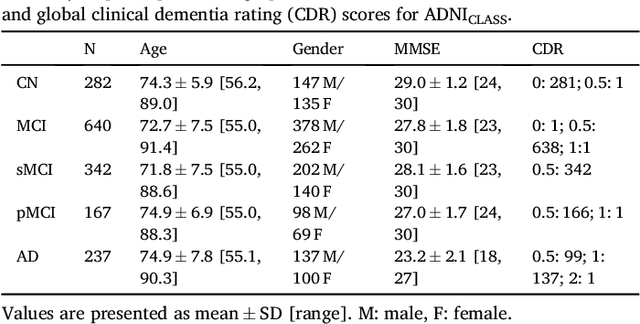
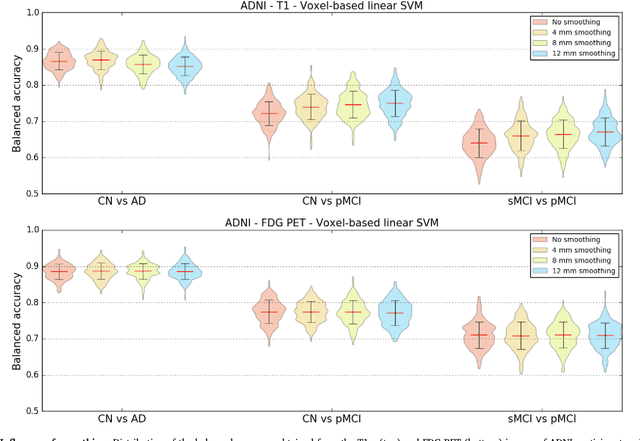
Abstract:A large number of papers have introduced novel machine learning and feature extraction methods for automatic classification of AD. However, they are difficult to reproduce because key components of the validation are often not readily available. These components include selected participants and input data, image preprocessing and cross-validation procedures. The performance of the different approaches is also difficult to compare objectively. In particular, it is often difficult to assess which part of the method provides a real improvement, if any. We propose a framework for reproducible and objective classification experiments in AD using three publicly available datasets (ADNI, AIBL and OASIS). The framework comprises: i) automatic conversion of the three datasets into BIDS format, ii) a modular set of preprocessing pipelines, feature extraction and classification methods, together with an evaluation framework, that provide a baseline for benchmarking the different components. We demonstrate the use of the framework for a large-scale evaluation on 1960 participants using T1 MRI and FDG PET data. In this evaluation, we assess the influence of different modalities, preprocessing, feature types, classifiers, training set sizes and datasets. Performances were in line with the state-of-the-art. FDG PET outperformed T1 MRI for all classification tasks. No difference in performance was found for the use of different atlases, image smoothing, partial volume correction of FDG PET images, or feature type. Linear SVM and L2-logistic regression resulted in similar performance and both outperformed random forests. The classification performance increased along with the number of subjects used for training. Classifiers trained on ADNI generalized well to AIBL and OASIS. All the code of the framework and the experiments is publicly available at: https://gitlab.icm-institute.org/aramislab/AD-ML.
Prediction of the progression of subcortical brain structures in Alzheimer's disease from baseline
Nov 23, 2017


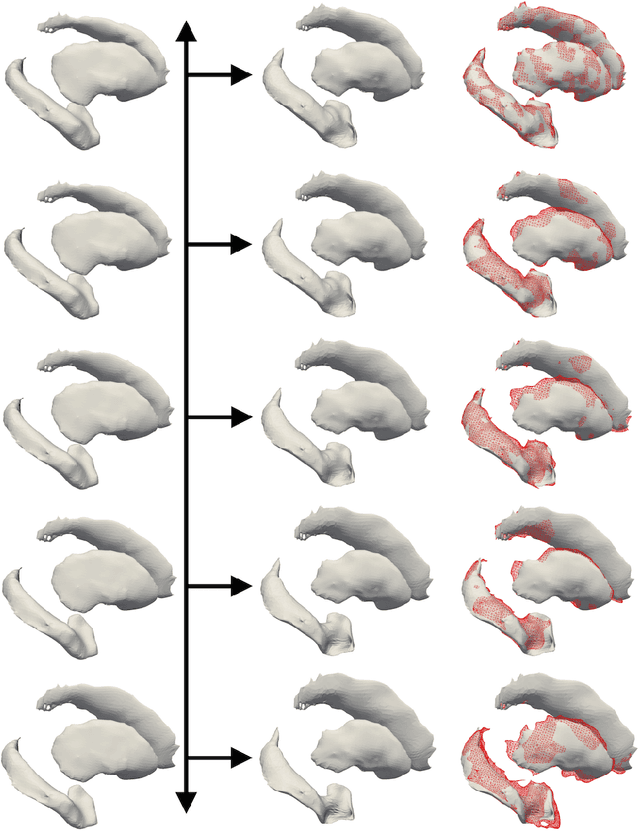
Abstract:We propose a method to predict the subject-specific longitudinal progression of brain structures extracted from baseline MRI, and evaluate its performance on Alzheimer's disease data. The disease progression is modeled as a trajectory on a group of diffeomorphisms in the context of large deformation diffeomorphic metric mapping (LDDMM). We first exhibit the limited predictive abilities of geodesic regression extrapolation on this group. Building on the recent concept of parallel curves in shape manifolds, we then introduce a second predictive protocol which personalizes previously learned trajectories to new subjects, and investigate the relative performances of two parallel shifting paradigms. This design only requires the baseline imaging data. Finally, coefficients encoding the disease dynamics are obtained from longitudinal cognitive measurements for each subject, and exploited to refine our methodology which is demonstrated to successfully predict the follow-up visits.
Statistical learning of spatiotemporal patterns from longitudinal manifold-valued networks
Sep 25, 2017


Abstract:We introduce a mixed-effects model to learn spatiotempo-ral patterns on a network by considering longitudinal measures distributed on a fixed graph. The data come from repeated observations of subjects at different time points which take the form of measurement maps distributed on a graph such as an image or a mesh. The model learns a typical group-average trajectory characterizing the propagation of measurement changes across the graph nodes. The subject-specific trajectories are defined via spatial and temporal transformations of the group-average scenario, thus estimating the variability of spatiotemporal patterns within the group. To estimate population and individual model parameters, we adapted a stochastic version of the Expectation-Maximization algorithm, the MCMC-SAEM. The model is used to describe the propagation of cortical atrophy during the course of Alzheimer's Disease. Model parameters show the variability of this average pattern of atrophy in terms of trajectories across brain regions, age at disease onset and pace of propagation. We show that the personalization of this model yields accurate prediction of maps of cortical thickness in patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge