Pascal Lu
Reproducible evaluation of classification methods in Alzheimer's disease: framework and application to MRI and PET data
Aug 20, 2018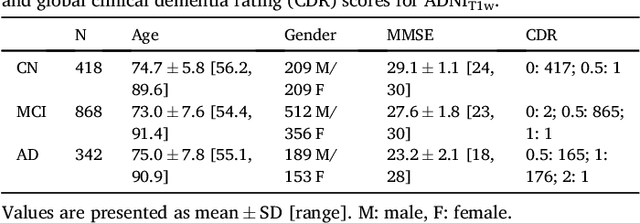
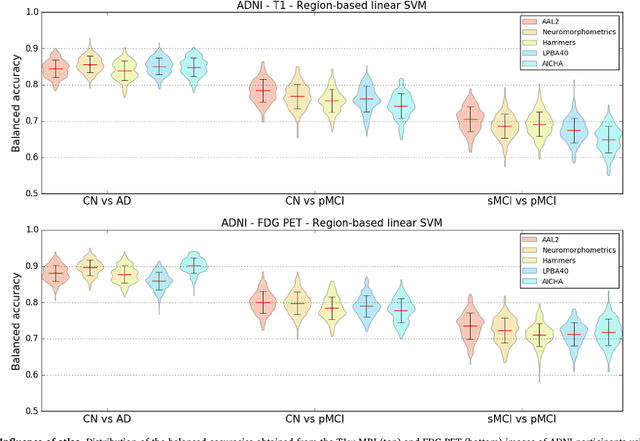
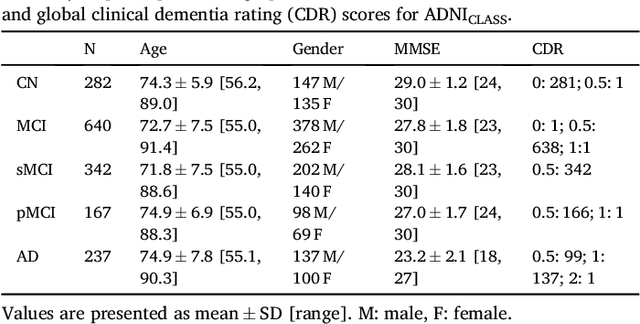
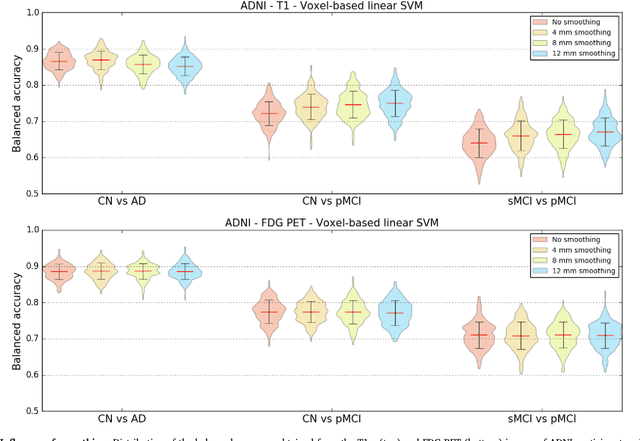
Abstract:A large number of papers have introduced novel machine learning and feature extraction methods for automatic classification of AD. However, they are difficult to reproduce because key components of the validation are often not readily available. These components include selected participants and input data, image preprocessing and cross-validation procedures. The performance of the different approaches is also difficult to compare objectively. In particular, it is often difficult to assess which part of the method provides a real improvement, if any. We propose a framework for reproducible and objective classification experiments in AD using three publicly available datasets (ADNI, AIBL and OASIS). The framework comprises: i) automatic conversion of the three datasets into BIDS format, ii) a modular set of preprocessing pipelines, feature extraction and classification methods, together with an evaluation framework, that provide a baseline for benchmarking the different components. We demonstrate the use of the framework for a large-scale evaluation on 1960 participants using T1 MRI and FDG PET data. In this evaluation, we assess the influence of different modalities, preprocessing, feature types, classifiers, training set sizes and datasets. Performances were in line with the state-of-the-art. FDG PET outperformed T1 MRI for all classification tasks. No difference in performance was found for the use of different atlases, image smoothing, partial volume correction of FDG PET images, or feature type. Linear SVM and L2-logistic regression resulted in similar performance and both outperformed random forests. The classification performance increased along with the number of subjects used for training. Classifiers trained on ADNI generalized well to AIBL and OASIS. All the code of the framework and the experiments is publicly available at: https://gitlab.icm-institute.org/aramislab/AD-ML.
Multilevel Modeling with Structured Penalties for Classification from Imaging Genetics data
Oct 10, 2017


Abstract:In this paper, we propose a framework for automatic classification of patients from multimodal genetic and brain imaging data by optimally combining them. Additive models with unadapted penalties (such as the classical group lasso penalty or $L_1$-multiple kernel learning) treat all modalities in the same manner and can result in undesirable elimination of specific modalities when their contributions are unbalanced. To overcome this limitation, we introduce a multilevel model that combines imaging and genetics and that considers joint effects between these two modalities for diagnosis prediction. Furthermore, we propose a framework allowing to combine several penalties taking into account the structure of the different types of data, such as a group lasso penalty over the genetic modality and a $L_2$-penalty on imaging modalities. Finally , we propose a fast optimization algorithm, based on a proximal gradient method. The model has been evaluated on genetic (single nucleotide polymorphisms-SNP) and imaging (anatomical MRI measures) data from the ADNI database, and compared to additive models. It exhibits good performances in AD diagnosis; and at the same time, reveals relationships between genes, brain regions and the disease status.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge