Max-Heinrich Laves
Posterior temperature optimized Bayesian models for inverse problems in medical imaging
Feb 02, 2022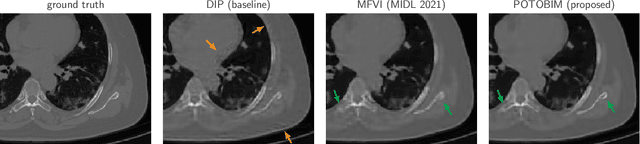
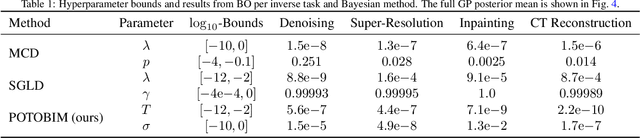
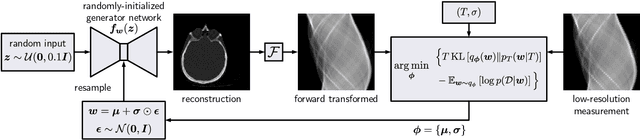
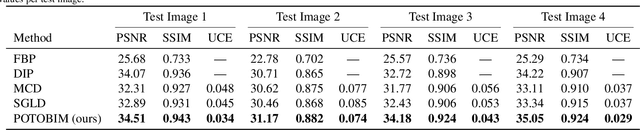
Abstract:We present Posterior Temperature Optimized Bayesian Inverse Models (POTOBIM), an unsupervised Bayesian approach to inverse problems in medical imaging using mean-field variational inference with a fully tempered posterior. Bayesian methods exhibit useful properties for approaching inverse tasks, such as tomographic reconstruction or image denoising. A suitable prior distribution introduces regularization, which is needed to solve the ill-posed problem and reduces overfitting the data. In practice, however, this often results in a suboptimal posterior temperature, and the full potential of the Bayesian approach is not being exploited. In POTOBIM, we optimize both the parameters of the prior distribution and the posterior temperature with respect to reconstruction accuracy using Bayesian optimization with Gaussian process regression. Our method is extensively evaluated on four different inverse tasks on a variety of modalities with images from public data sets and we demonstrate that an optimized posterior temperature outperforms both non-Bayesian and Bayesian approaches without temperature optimization. The use of an optimized prior distribution and posterior temperature leads to improved accuracy and uncertainty estimation and we show that it is sufficient to find these hyperparameters per task domain. Well-tempered posteriors yield calibrated uncertainty, which increases the reliability in the predictions. Our source code is publicly available at github.com/Cardio-AI/mfvi-dip-mia.
Unsupervised Anomaly Detection in 3D Brain MRI using Deep Learning with Multi-Task Brain Age Prediction
Jan 31, 2022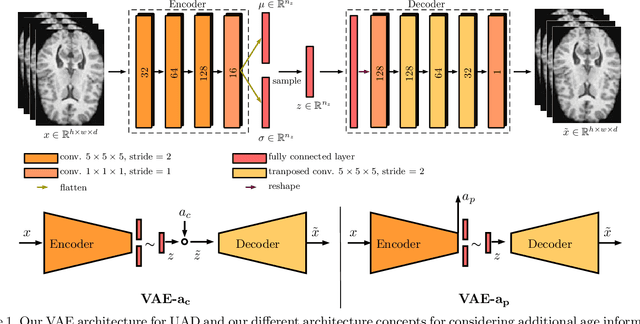

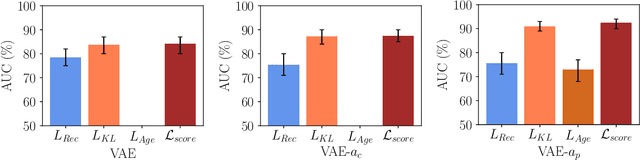
Abstract:Lesion detection in brain Magnetic Resonance Images (MRIs) remains a challenging task. MRIs are typically read and interpreted by domain experts, which is a tedious and time-consuming process. Recently, unsupervised anomaly detection (UAD) in brain MRI with deep learning has shown promising results to provide a quick, initial assessment. So far, these methods only rely on the visual appearance of healthy brain anatomy for anomaly detection. Another biomarker for abnormal brain development is the deviation between the brain age and the chronological age, which is unexplored in combination with UAD. We propose deep learning for UAD in 3D brain MRI considering additional age information. We analyze the value of age information during training, as an additional anomaly score, and systematically study several architecture concepts. Based on our analysis, we propose a novel deep learning approach for UAD with multi-task age prediction. We use clinical T1-weighted MRIs of 1735 healthy subjects and the publicly available BraTs 2019 data set for our study. Our novel approach significantly improves UAD performance with an AUC of 92.60% compared to an AUC-score of 84.37% using previous approaches without age information.
Robotic Tissue Sampling for Safe Post-mortem Biopsy in Infectious Corpses
Jan 28, 2022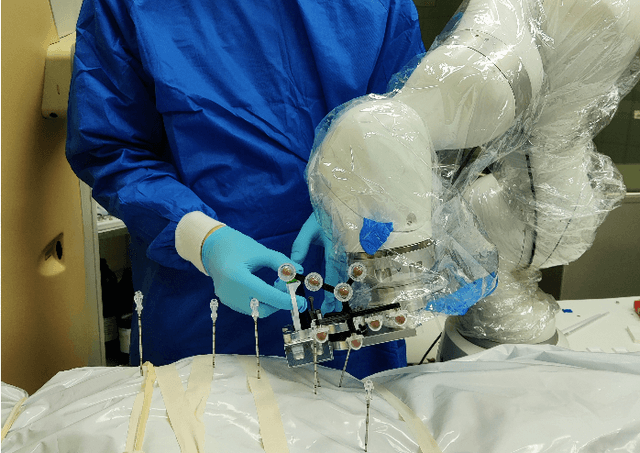
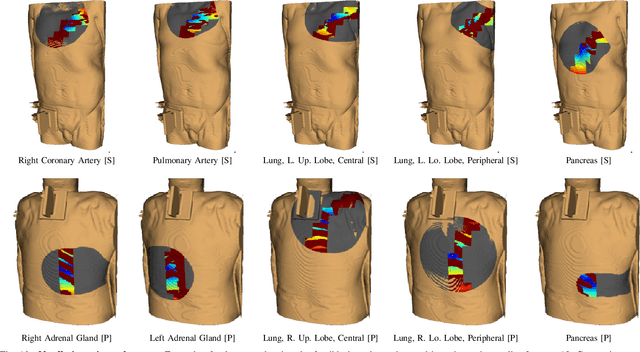
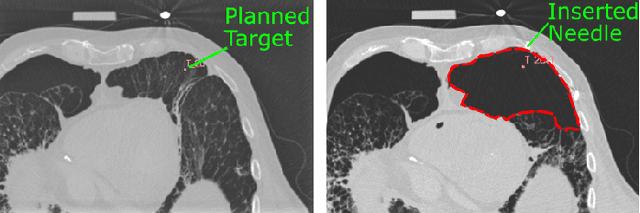
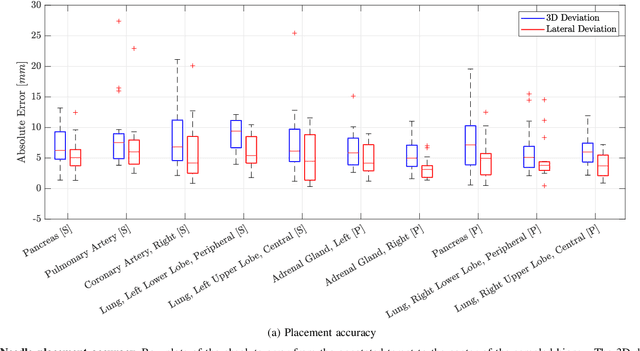
Abstract:In pathology and legal medicine, the histopathological and microbiological analysis of tissue samples from infected deceased is a valuable information for developing treatment strategies during a pandemic such as COVID-19. However, a conventional autopsy carries the risk of disease transmission and may be rejected by relatives. We propose minimally invasive biopsy with robot assistance under CT guidance to minimize the risk of disease transmission during tissue sampling and to improve accuracy. A flexible robotic system for biopsy sampling is presented, which is applied to human corpses placed inside protective body bags. An automatic planning and decision system estimates optimal insertion point. Heat maps projected onto the segmented skin visualize the distance and angle of insertions and estimate the minimum cost of a puncture while avoiding bone collisions. Further, we test multiple insertion paths concerning feasibility and collisions. A custom end effector is designed for inserting needles and extracting tissue samples under robotic guidance. Our robotic post-mortem biopsy (RPMB) system is evaluated in a study during the COVID-19 pandemic on 20 corpses and 10 tissue targets, 5 of them being infected with SARS-CoV-2. The mean planning time including robot path planning is (5.72+-1.67) s. Mean needle placement accuracy is (7.19+-4.22) mm.
Cold Posteriors Improve Bayesian Medical Image Post-Processing
Jul 12, 2021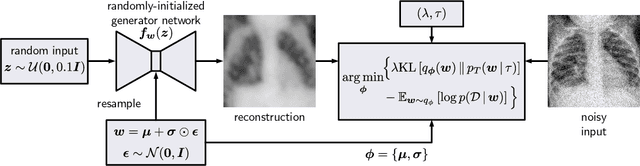
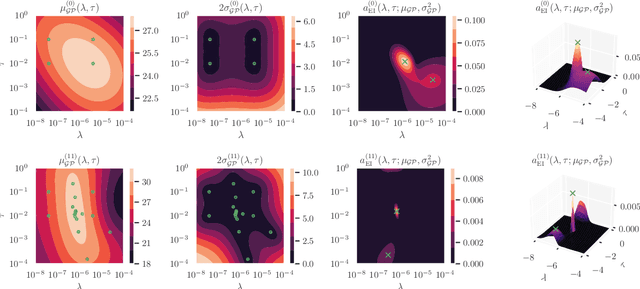
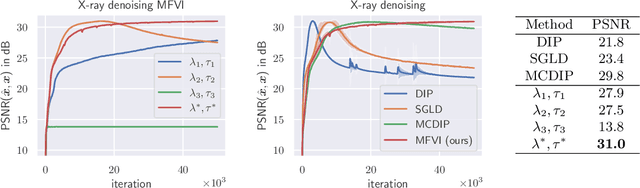
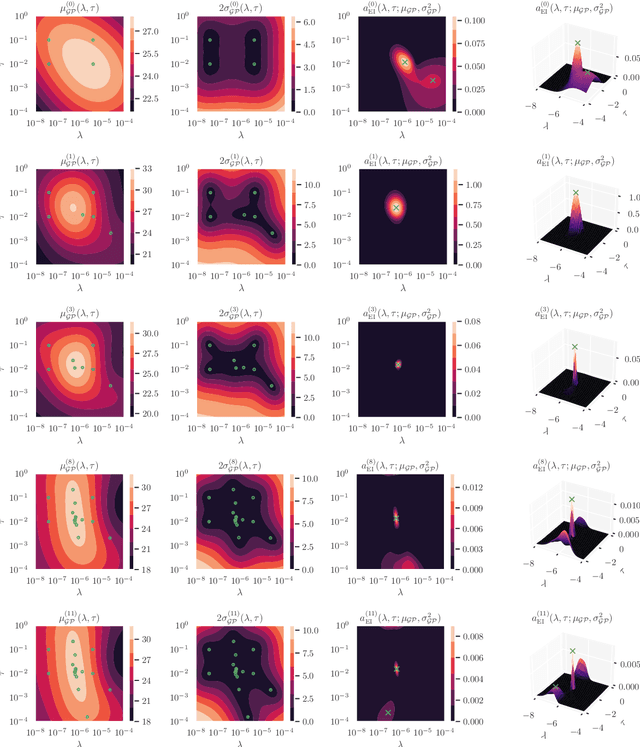
Abstract:Cold posteriors have been reported to perform better in practice in the context of Bayesian deep learning (Wenzel et al., 2020). In variational inference, it is common to employ only a partially tempered posterior by scaling the complexity term in the log-evidence lower bound (ELBO). In this work, we optimize the ELBO for a fully tempered posterior in mean-field variational inference and use Bayesian optimization to automatically find the optimal posterior temperature and prior scale. Choosing an appropriate posterior temperature leads to better predictive performance and improved uncertainty calibration, which we demonstrate for the task of denoising medical X-ray images.
Recalibration of Aleatoric and Epistemic Regression Uncertainty in Medical Imaging
Apr 26, 2021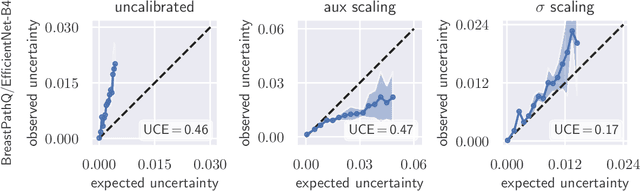
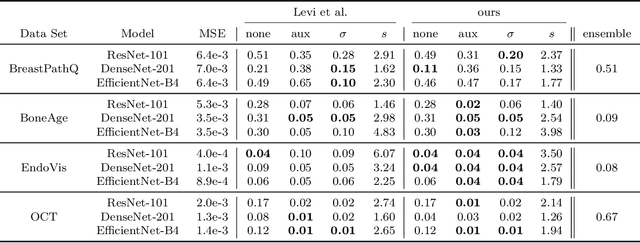
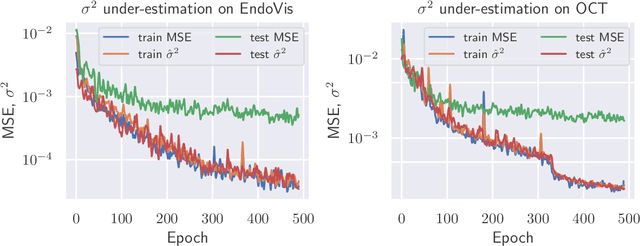
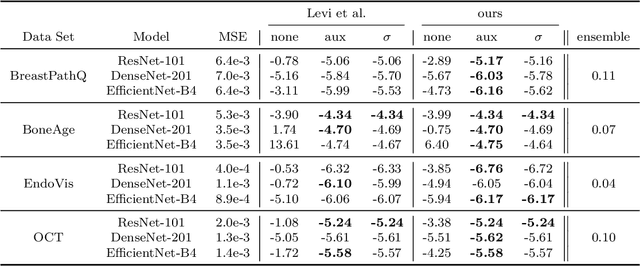
Abstract:The consideration of predictive uncertainty in medical imaging with deep learning is of utmost importance. We apply estimation of both aleatoric and epistemic uncertainty by variational Bayesian inference with Monte Carlo dropout to regression tasks and show that predictive uncertainty is systematically underestimated. We apply $ \sigma $ scaling with a single scalar value; a simple, yet effective calibration method for both types of uncertainty. The performance of our approach is evaluated on a variety of common medical regression data sets using different state-of-the-art convolutional network architectures. In our experiments, $ \sigma $ scaling is able to reliably recalibrate predictive uncertainty. It is easy to implement and maintains the accuracy. Well-calibrated uncertainty in regression allows robust rejection of unreliable predictions or detection of out-of-distribution samples. Our source code is available at https://github.com/mlaves/well-calibrated-regression-uncertainty
Uncertainty Estimation in Medical Image Denoising with Bayesian Deep Image Prior
Aug 20, 2020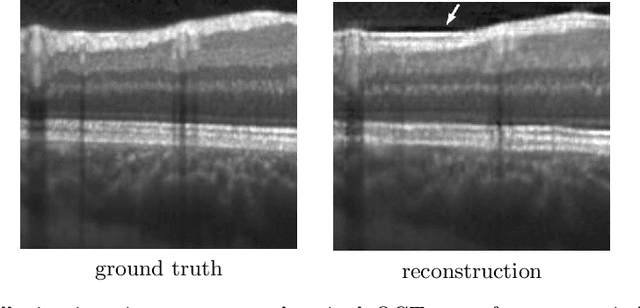
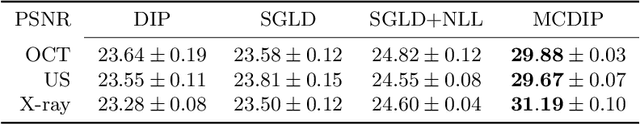
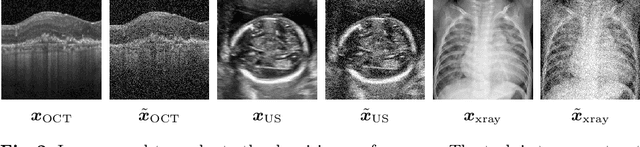

Abstract:Uncertainty quantification in inverse medical imaging tasks with deep learning has received little attention. However, deep models trained on large data sets tend to hallucinate and create artifacts in the reconstructed output that are not anatomically present. We use a randomly initialized convolutional network as parameterization of the reconstructed image and perform gradient descent to match the observation, which is known as deep image prior. In this case, the reconstruction does not suffer from hallucinations as no prior training is performed. We extend this to a Bayesian approach with Monte Carlo dropout to quantify both aleatoric and epistemic uncertainty. The presented method is evaluated on the task of denoising different medical imaging modalities. The experimental results show that our approach yields well-calibrated uncertainty. That is, the predictive uncertainty correlates with the predictive error. This allows for reliable uncertainty estimates and can tackle the problem of hallucinations and artifacts in inverse medical imaging tasks.
Patient-Specific Domain Adaptation for Fast Optical Flow Based on Teacher-Student Knowledge Transfer
Jul 09, 2020
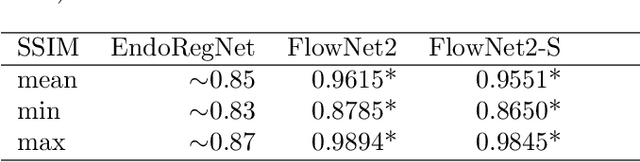
Abstract:Fast motion feedback is crucial in computer-aided surgery (CAS) on moving tissue. Image-assistance in safety-critical vision applications requires a dense tracking of tissue motion. This can be done using optical flow (OF). Accurate motion predictions at high processing rates lead to higher patient safety. Current deep learning OF models show the common speed vs. accuracy trade-off. To achieve high accuracy at high processing rates, we propose patient-specific fine-tuning of a fast model. This minimizes the domain gap between training and application data, while reducing the target domain to the capability of the lower complex, fast model. We propose to obtain training sequences pre-operatively in the operation room. We handle missing ground truth, by employing teacher-student learning. Using flow estimations from teacher model FlowNet2 we specialize a fast student model FlowNet2S on the patient-specific domain. Evaluation is performed on sequences from the Hamlyn dataset. Our student model shows very good performance after fine-tuning. Tracking accuracy is comparable to the teacher model at a speed up of factor six. Fine-tuning can be performed within minutes, making it feasible for the operation room. Our method allows to use a real-time capable model that was previously not suited for this task. This method is laying the path for improved patient-specific motion estimation in CAS.
Calibration of Model Uncertainty for Dropout Variational Inference
Jun 20, 2020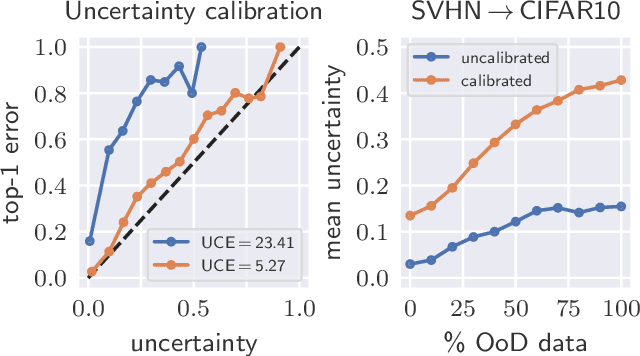
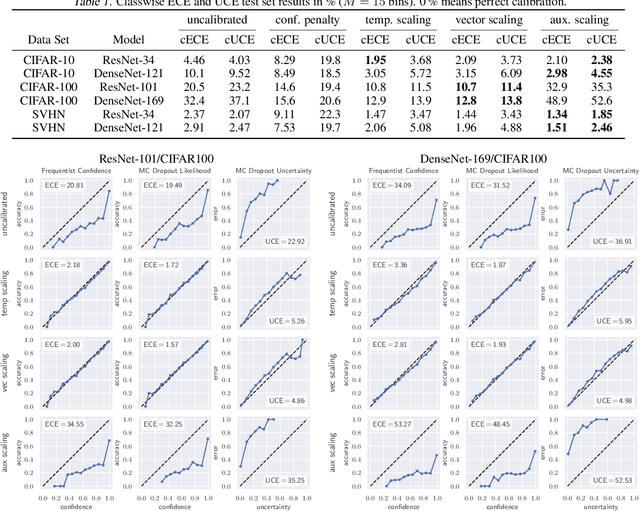
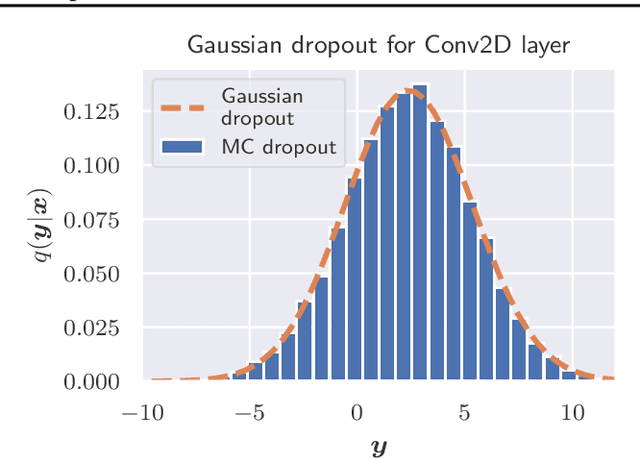
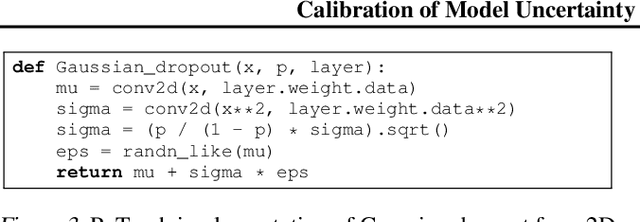
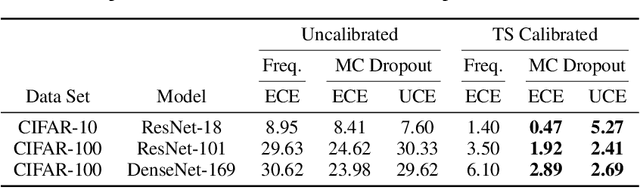
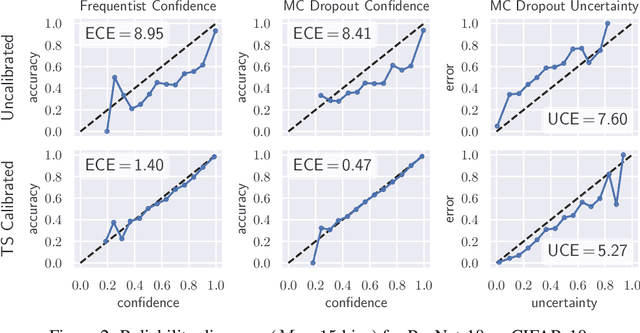
Abstract:The model uncertainty obtained by variational Bayesian inference with Monte Carlo dropout is prone to miscalibration. In this paper, different logit scaling methods are extended to dropout variational inference to recalibrate model uncertainty. Expected uncertainty calibration error (UCE) is presented as a metric to measure miscalibration. The effectiveness of recalibration is evaluated on CIFAR-10/100 and SVHN for recent CNN architectures. Experimental results show that logit scaling considerably reduce miscalibration by means of UCE. Well-calibrated uncertainty enables reliable rejection of uncertain predictions and robust detection of out-of-distribution data.
Well-calibrated Model Uncertainty with Temperature Scaling for Dropout Variational Inference
Oct 01, 2019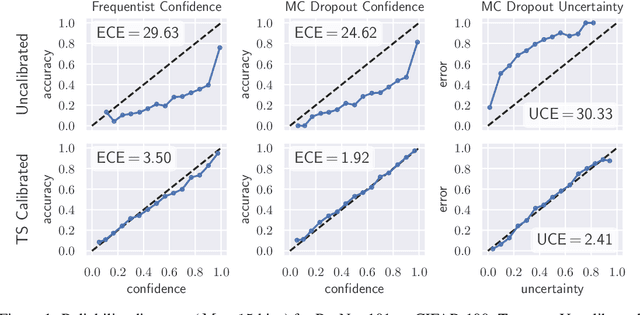
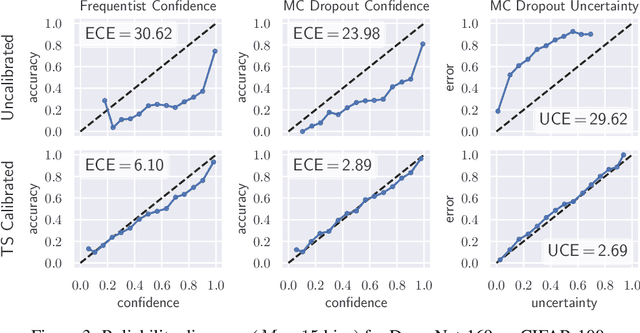
Abstract:In this paper, well-calibrated model uncertainty is obtained by using temperature scaling together with Monte Carlo dropout as approximation to Bayesian inference. The proposed approach can easily be derived from frequentist temperature scaling and yields well-calibrated model uncertainty as well as softmax likelihood.
Uncertainty Quantification in Computer-Aided Diagnosis: Make Your Model say "I don't know" for Ambiguous Cases
Aug 02, 2019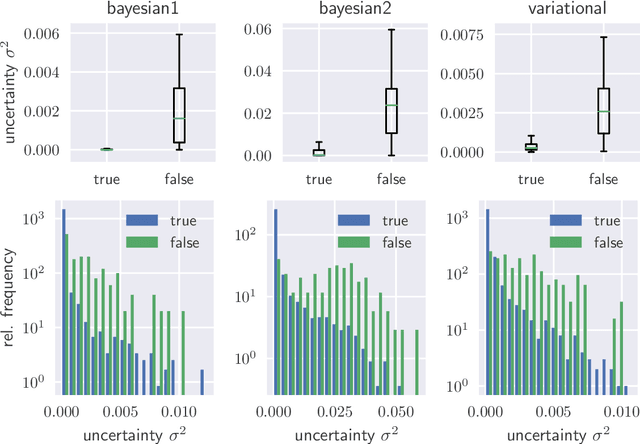

Abstract:We evaluate two different methods for the integration of prediction uncertainty into diagnostic image classifiers to increase patient safety in deep learning. In the first method, Monte Carlo sampling is applied with dropout at test time to get a posterior distribution of the class labels (Bayesian ResNet). The second method extends ResNet to a probabilistic approach by predicting the parameters of the posterior distribution and sampling the final result from it (Variational ResNet).The variance of the posterior is used as metric for uncertainty.Both methods are trained on a data set of optical coherence tomography scans showing four different retinal conditions. Our results shown that cases in which the classifier predicts incorrectly correlate with a higher uncertainty. Mean uncertainty of incorrectly diagnosed cases was between 4.6 and 8.1 times higher than mean uncertainty of correctly diagnosed cases. Modeling of the prediction uncertainty in computer-aided diagnosis with deep learning yields more reliable results and is anticipated to increase patient safety.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge