Justin Blaber
MRI correlates of chronic symptoms in mild traumatic brain injury
Dec 06, 2019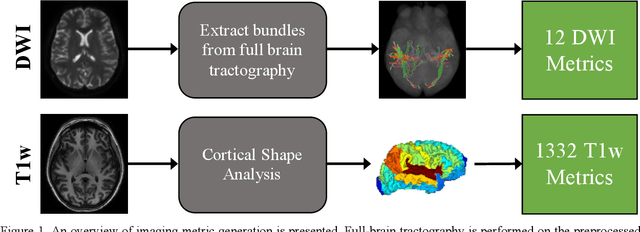
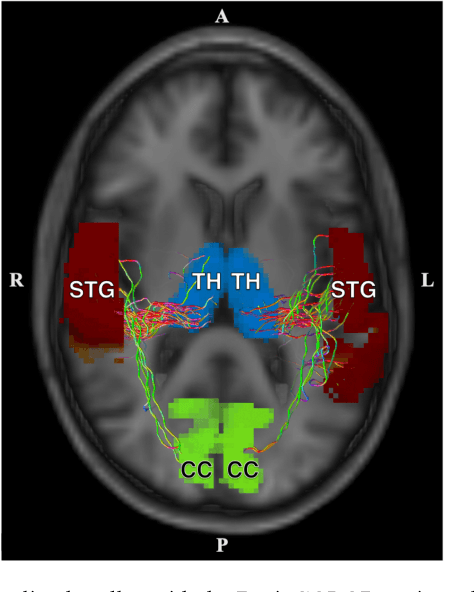
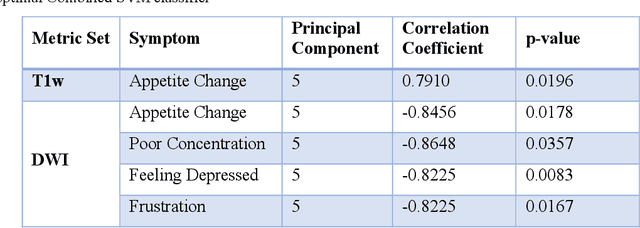
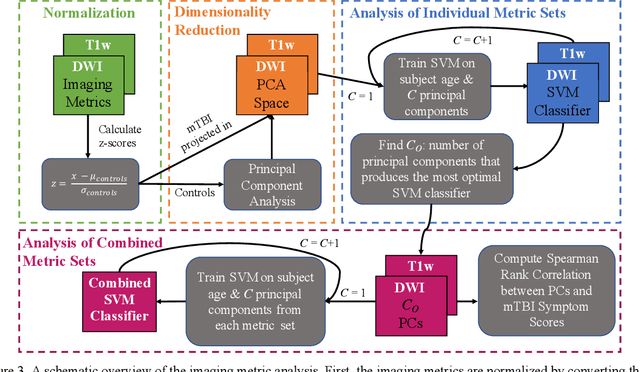
Abstract:Veterans with mild traumatic brain injury (mTBI) have reported auditory and visual dysfunction that persists beyond the acute incident. The etiology behind these symptoms is difficult to characterize with current clinical imaging. These functional deficits may be caused by shear injury or micro-bleeds, which can be detected with special imaging modalities. We explore these hypotheses in a pilot study of multi-parametric MRI. We extract over 1,000 imaging and clinical metrics and project them to a low-dimensional space, where we can discriminate between healthy controls and patients with mTBI. We also show correlations between the metric representations and patient symptoms.
Generalizing Deep Whole Brain Segmentation for Pediatric and Post-Contrast MRI with Augmented Transfer Learning
Aug 13, 2019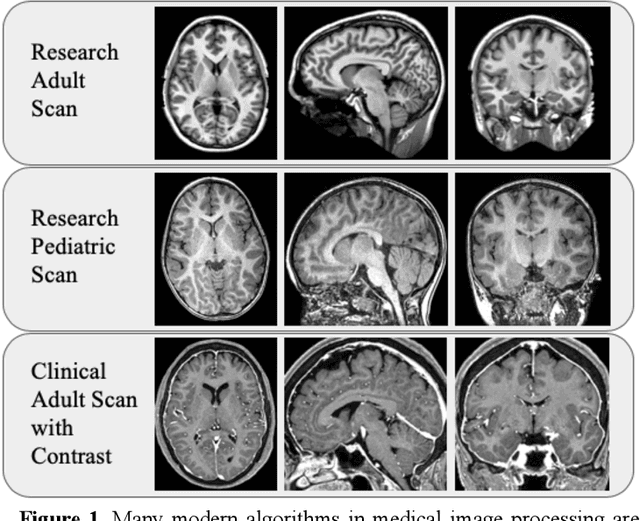
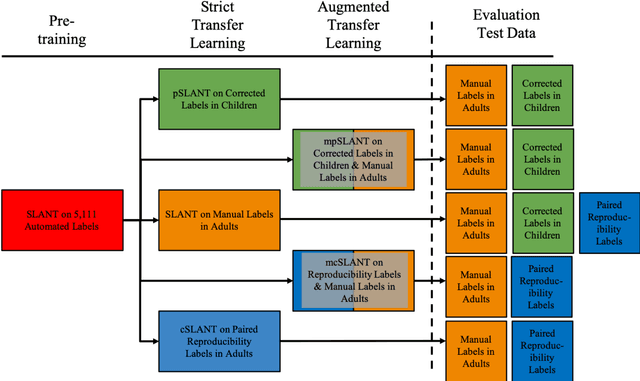

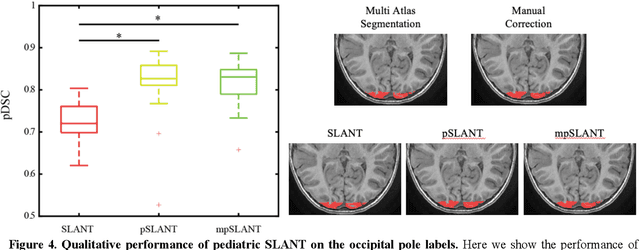
Abstract:Generalizability is an important problem in deep neural networks, especially in the context of the variability of data acquisition in clinical magnetic resonance imaging (MRI). Recently, the Spatially Localized Atlas Network Tiles (SLANT) approach has been shown to effectively segment whole brain non-contrast T1w MRI with 132 volumetric labels. Enhancing generalizability of SLANT would enable broader application of volumetric assessment in multi-site studies. Transfer learning (TL) is commonly used to update the neural network weights for local factors; yet, it is commonly recognized to risk degradation of performance on the original validation/test cohorts. Here, we explore TL by data augmentation to address these concerns in the context of adapting SLANT to anatomical variation and scanning protocol. We consider two datasets: First, we optimize for age with 30 T1w MRI of young children with manually corrected volumetric labels, and accuracy of automated segmentation defined relative to the manually provided truth. Second, we optimize for acquisition with 36 paired datasets of pre- and post-contrast clinically acquired T1w MRI, and accuracy of the post-contrast segmentations assessed relative to the pre-contrast automated assessment. For both studies, we augment the original TL step of SLANT with either only the new data or with both original and new data. Over baseline SLANT, both approaches yielded significantly improved performance (signed rank tests; pediatric: 0.89 vs. 0.82 DSC, p<0.001; contrast: 0.80 vs 0.76, p<0.001). The performance on the original test set decreased with the new-data only transfer learning approach, so data augmentation was superior to strict transfer learning.
Distributed deep learning for robust multi-site segmentation of CT imaging after traumatic brain injury
Mar 11, 2019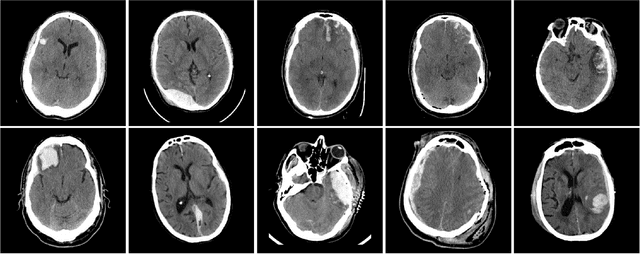

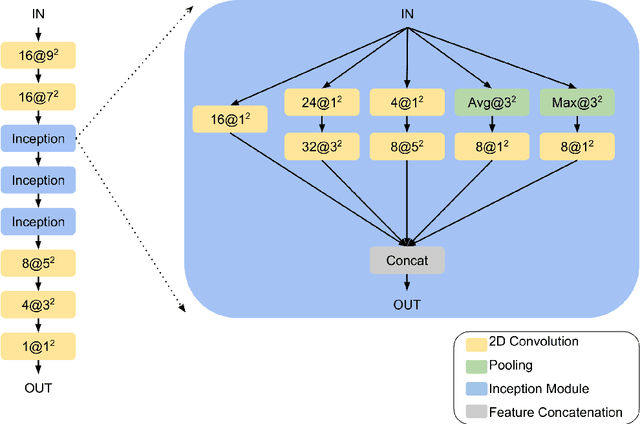
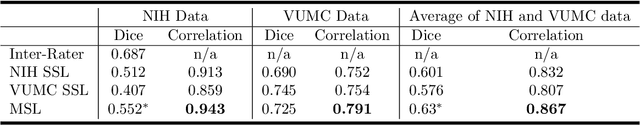
Abstract:Machine learning models are becoming commonplace in the domain of medical imaging, and with these methods comes an ever-increasing need for more data. However, to preserve patient anonymity it is frequently impractical or prohibited to transfer protected health information (PHI) between institutions. Additionally, due to the nature of some studies, there may not be a large public dataset available on which to train models. To address this conundrum, we analyze the efficacy of transferring the model itself in lieu of data between different sites. By doing so we accomplish two goals: 1) the model gains access to training on a larger dataset that it could not normally obtain and 2) the model better generalizes, having trained on data from separate locations. In this paper, we implement multi-site learning with disparate datasets from the National Institutes of Health (NIH) and Vanderbilt University Medical Center (VUMC) without compromising PHI. Three neural networks are trained to convergence on a computed tomography (CT) brain hematoma segmentation task: one only with NIH data,one only with VUMC data, and one multi-site model alternating between NIH and VUMC data. Resultant lesion masks with the multi-site model attain an average Dice similarity coefficient of 0.64 and the automatically segmented hematoma volumes correlate to those done manually with a Pearson correlation coefficient of 0.87,corresponding to an 8% and 5% improvement, respectively, over the single-site model counterparts.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge