Jiahui Guan
Development and Validation of a Deep Learning Model for Prediction of Severe Outcomes in Suspected COVID-19 Infection
Mar 29, 2021



Abstract:COVID-19 patient triaging with predictive outcome of the patients upon first present to emergency department (ED) is crucial for improving patient prognosis, as well as better hospital resources management and cross-infection control. We trained a deep feature fusion model to predict patient outcomes, where the model inputs were EHR data including demographic information, co-morbidities, vital signs and laboratory measurements, plus patient's CXR images. The model output was patient outcomes defined as the most insensitive oxygen therapy required. For patients without CXR images, we employed Random Forest method for the prediction. Predictive risk scores for COVID-19 severe outcomes ("CO-RISK" score) were derived from model output and evaluated on the testing dataset, as well as compared to human performance. The study's dataset (the "MGB COVID Cohort") was constructed from all patients presenting to the Mass General Brigham (MGB) healthcare system from March 1st to June 1st, 2020. ED visits with incomplete or erroneous data were excluded. Patients with no test order for COVID or confirmed negative test results were excluded. Patients under the age of 15 were also excluded. Finally, electronic health record (EHR) data from a total of 11060 COVID-19 confirmed or suspected patients were used in this study. Chest X-ray (CXR) images were also collected from each patient if available. Results show that CO-RISK score achieved area under the Curve (AUC) of predicting MV/death (i.e. severe outcomes) in 24 hours of 0.95, and 0.92 in 72 hours on the testing dataset. The model shows superior performance to the commonly used risk scores in ED (CURB-65 and MEWS). Comparing with physician's decisions, CO-RISK score has demonstrated superior performance to human in making ICU/floor decisions.
Impact of Inference Accelerators on hardware selection
Oct 07, 2019



Abstract:As opportunities for AI-assisted healthcare grow steadily, model deployment faces challenges due to the specific characteristics of the industry. The configuration choice for a production device can impact model performance while influencing operational costs. Moreover, in healthcare some situations might require fast, but not real time, inference. We study different configurations and conduct a cost-performance analysis to determine the optimized hardware for the deployment of a model subject to healthcare domain constraints. We observe that a naive performance comparison may not lead to an optimal configuration selection. In fact, given realistic domain constraints, CPU execution might be preferable to GPU accelerators. Hence, defining beforehand precise expectations for model deployment is crucial.
Iterative PET Image Reconstruction Using Convolutional Neural Network Representation
Oct 09, 2017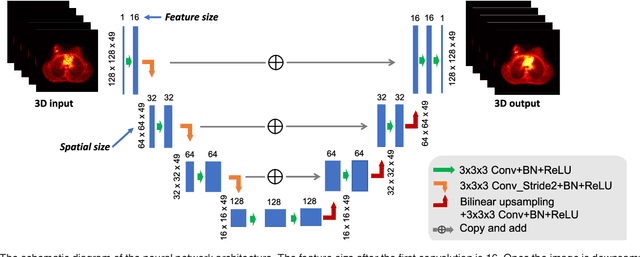
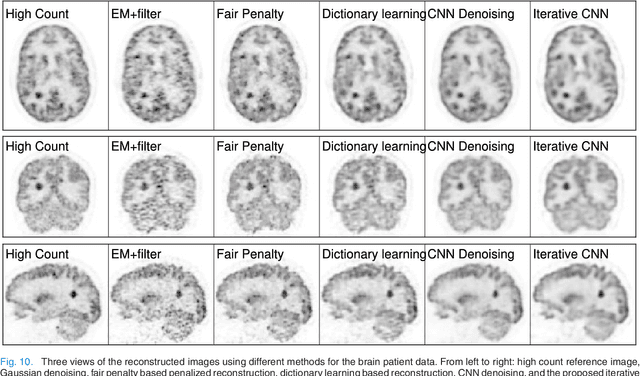
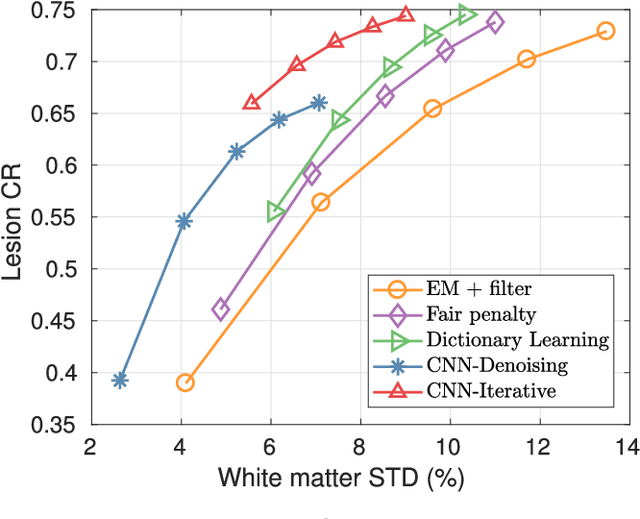
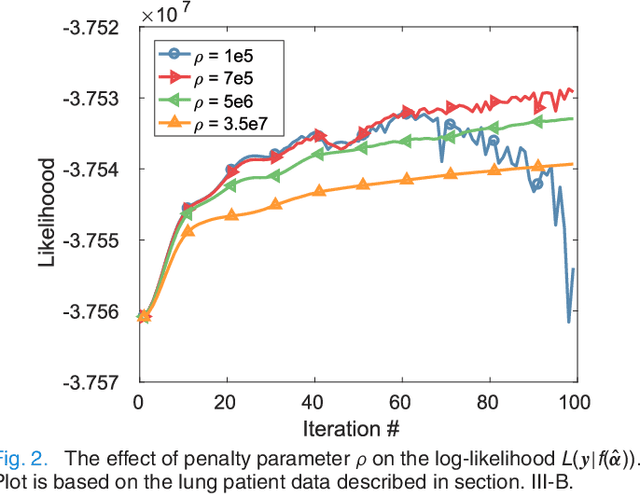
Abstract:PET image reconstruction is challenging due to the ill-poseness of the inverse problem and limited number of detected photons. Recently deep neural networks have been widely and successfully used in computer vision tasks and attracted growing interests in medical imaging. In this work, we trained a deep residual convolutional neural network to improve PET image quality by using the existing inter-patient information. An innovative feature of the proposed method is that we embed the neural network in the iterative reconstruction framework for image representation, rather than using it as a post-processing tool. We formulate the objective function as a constraint optimization problem and solve it using the alternating direction method of multipliers (ADMM) algorithm. Both simulation data and hybrid real data are used to evaluate the proposed method. Quantification results show that our proposed iterative neural network method can outperform the neural network denoising and conventional penalized maximum likelihood methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge