Ivo Baltruschat
Similarity Metrics for MR Image-To-Image Translation
May 15, 2024Abstract:Image-to-image translation can create large impact in medical imaging, i.e. if images of a patient can be translated to another modality, type or sequence for better diagnosis. However, these methods must be validated by human reader studies, which are costly and restricted to small samples. Automatic evaluation of large samples to pre-evaluate and continuously improve methods before human validation is needed. In this study, we give an overview of reference and non-reference metrics for image synthesis assessment and investigate the ability of nine metrics, that need a reference (SSIM, MS-SSIM, PSNR, MSE, NMSE, MAE, LPIPS, NMI and PCC) and three non-reference metrics (BLUR, MSN, MNG) to detect 11 kinds of distortions in MR images from the BraSyn dataset. In addition we test a downstream segmentation metric and the effect of three normalization methods (Minmax, cMinMax and Zscore). Although PSNR and SSIM are frequently used to evaluate generative models for image-to-image-translation tasks in the medical domain, they show very specific shortcomings. SSIM ignores blurring but is very sensitive to intensity shifts in unnormalized MR images. PSNR is even more sensitive to different normalization methods and hardly measures the degree of distortions. Further metrics, such as LPIPS, NMI and DICE can be very useful to evaluate other similarity aspects. If the images to be compared are misaligned, most metrics are flawed. By carefully selecting and reasonably combining image similarity metrics, the training and selection of generative models for MR image synthesis can be improved. Many aspects of their output can be validated before final and costly evaluation by trained radiologists is conducted.
Surgical tool classification and localization: results and methods from the MICCAI 2022 SurgToolLoc challenge
May 11, 2023



Abstract:The ability to automatically detect and track surgical instruments in endoscopic videos can enable transformational interventions. Assessing surgical performance and efficiency, identifying skilled tool use and choreography, and planning operational and logistical aspects of OR resources are just a few of the applications that could benefit. Unfortunately, obtaining the annotations needed to train machine learning models to identify and localize surgical tools is a difficult task. Annotating bounding boxes frame-by-frame is tedious and time-consuming, yet large amounts of data with a wide variety of surgical tools and surgeries must be captured for robust training. Moreover, ongoing annotator training is needed to stay up to date with surgical instrument innovation. In robotic-assisted surgery, however, potentially informative data like timestamps of instrument installation and removal can be programmatically harvested. The ability to rely on tool installation data alone would significantly reduce the workload to train robust tool-tracking models. With this motivation in mind we invited the surgical data science community to participate in the challenge, SurgToolLoc 2022. The goal was to leverage tool presence data as weak labels for machine learning models trained to detect tools and localize them in video frames with bounding boxes. We present the results of this challenge along with many of the team's efforts. We conclude by discussing these results in the broader context of machine learning and surgical data science. The training data used for this challenge consisting of 24,695 video clips with tool presence labels is also being released publicly and can be accessed at https://console.cloud.google.com/storage/browser/isi-surgtoolloc-2022.
Deep Learning for Pneumothorax Detection and Localization in Chest Radiographs
Jul 16, 2019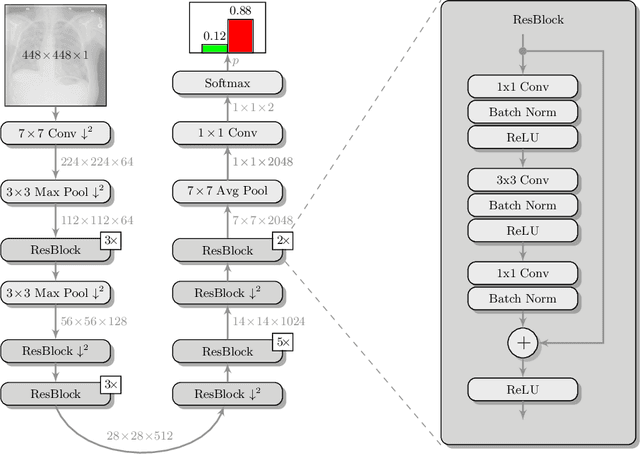
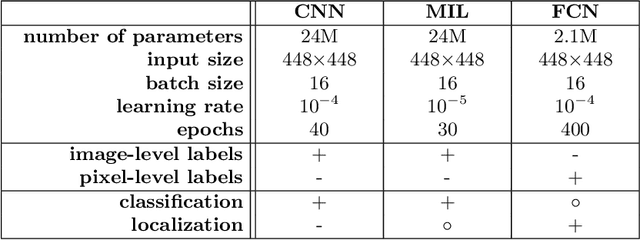
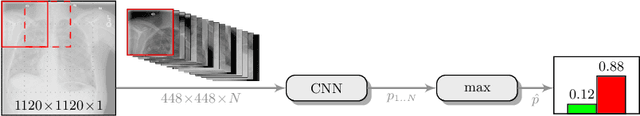
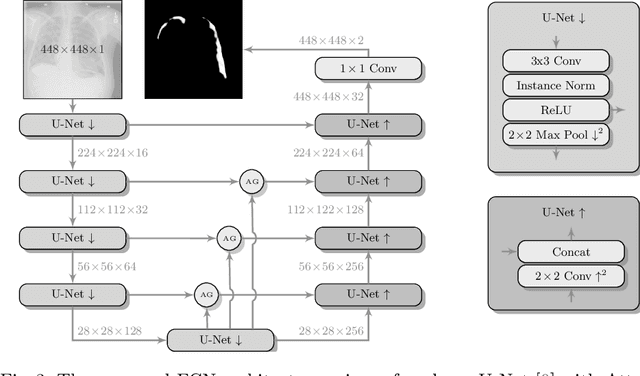
Abstract:Pneumothorax is a critical condition that requires timely communication and immediate action. In order to prevent significant morbidity or patient death, early detection is crucial. For the task of pneumothorax detection, we study the characteristics of three different deep learning techniques: (i) convolutional neural networks, (ii) multiple-instance learning, and (iii) fully convolutional networks. We perform a five-fold cross-validation on a dataset consisting of 1003 chest X-ray images. ROC analysis yields AUCs of 0.96, 0.93, and 0.92 for the three methods, respectively. We review the classification and localization performance of these approaches as well as an ensemble of the three aforementioned techniques.
Skin Lesion Classification Using CNNs with Patch-Based Attention and Diagnosis-Guided Loss Weighting
May 09, 2019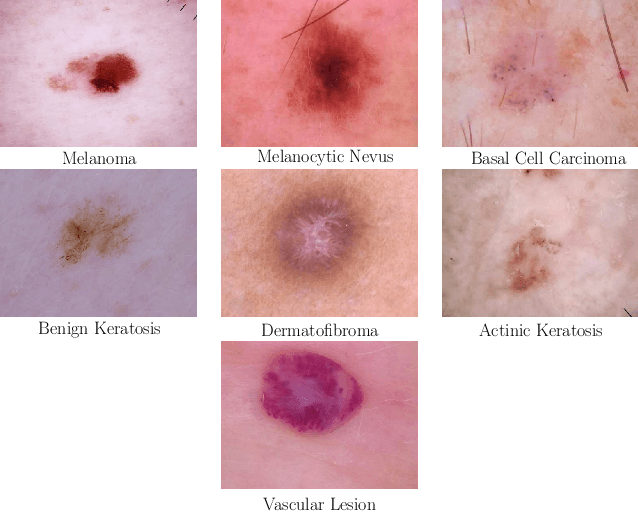
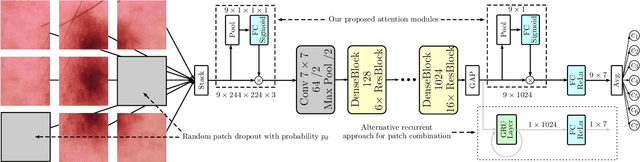
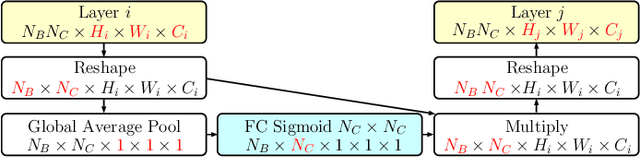
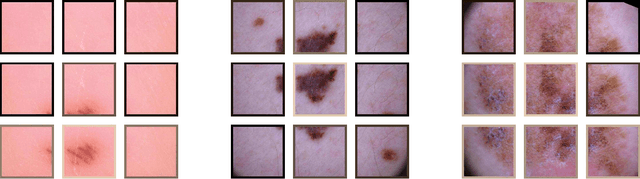
Abstract:Objective: This work addresses two key problems of skin lesion classification. The first problem is the effective use of high-resolution images with pretrained standard architectures for image classification. The second problem is the high class imbalance encountered in real-world multi-class datasets. Methods: To use high-resolution images, we propose a novel patch-based attention architecture that provides global context between small, high-resolution patches. We modify three pretrained architectures and study the performance of patch-based attention. To counter class imbalance problems, we compare oversampling, balanced batch sampling, and class-specific loss weighting. Additionally, we propose a novel diagnosis-guided loss weighting method which takes the method used for ground-truth annotation into account. Results: Our patch-based attention mechanism outperforms previous methods and improves the mean sensitivity by 7%. Class balancing significantly improves the mean sensitivity and we show that our diagnosis-guided loss weighting method improves the mean sensitivity by 3% over normal loss balancing. Conclusion: The novel patch-based attention mechanism can be integrated into pretrained architectures and provides global context between local patches while outperforming other patch-based methods. Hence, pretrained architectures can be readily used with high-resolution images without downsampling. The new diagnosis-guided loss weighting method outperforms other methods and allows for effective training when facing class imbalance. Significance: The proposed methods improve automatic skin lesion classification. They can be extended to other clinical applications where high-resolution image data and class imbalance are relevant.
Skin Lesion Diagnosis using Ensembles, Unscaled Multi-Crop Evaluation and Loss Weighting
Aug 05, 2018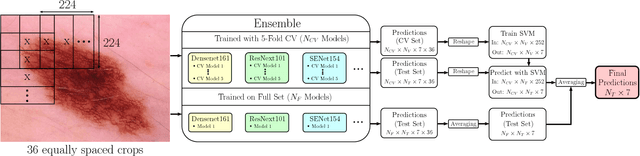
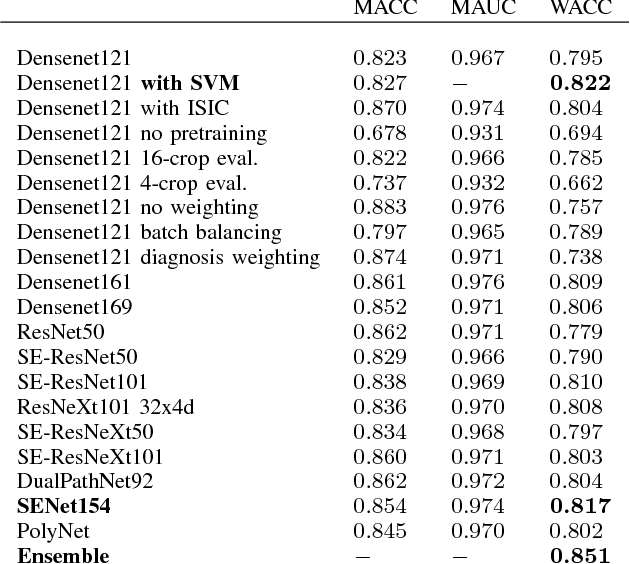
Abstract:In this paper we present the methods of our submission to the ISIC 2018 challenge for skin lesion diagnosis (Task 3). The dataset consists of 10000 images with seven image-level classes to be distinguished by an automated algorithm. We employ an ensemble of convolutional neural networks for this task. In particular, we fine-tune pretrained state-of-the-art deep learning models such as Densenet, SENet and ResNeXt. We identify heavy class imbalance as a key problem for this challenge and consider multiple balancing approaches such as loss weighting and balanced batch sampling. Another important feature of our pipeline is the use of a vast amount of unscaled crops for evaluation. Last, we consider meta learning approaches for the final predictions. Our team placed second at the challenge while being the best approach using only publicly available data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge