Guido Davidzon
Nuclear Medicine Artificial Intelligence in Action: The Bethesda Report (AI Summit 2024)
Jun 03, 2024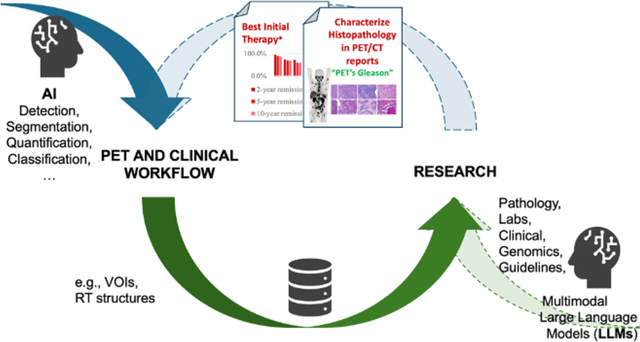
Abstract:The 2nd SNMMI Artificial Intelligence (AI) Summit, organized by the SNMMI AI Task Force, took place in Bethesda, MD, on February 29 - March 1, 2024. Bringing together various community members and stakeholders, and following up on a prior successful 2022 AI Summit, the summit theme was: AI in Action. Six key topics included (i) an overview of prior and ongoing efforts by the AI task force, (ii) emerging needs and tools for computational nuclear oncology, (iii) new frontiers in large language and generative models, (iv) defining the value proposition for the use of AI in nuclear medicine, (v) open science including efforts for data and model repositories, and (vi) issues of reimbursement and funding. The primary efforts, findings, challenges, and next steps are summarized in this manuscript.
Brain MRI-to-PET Synthesis using 3D Convolutional Attention Networks
Nov 22, 2022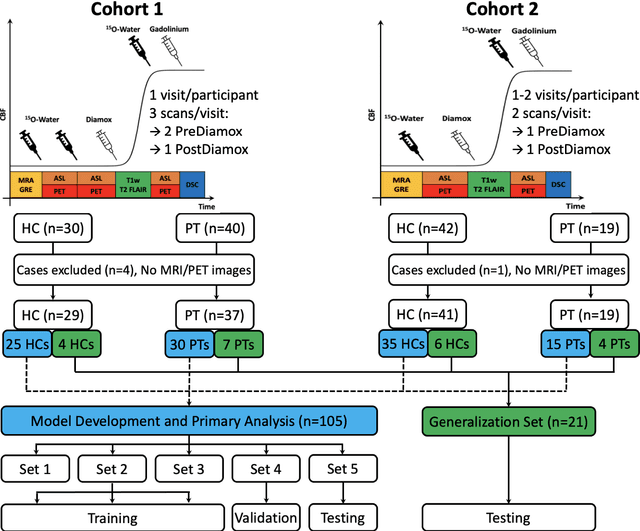
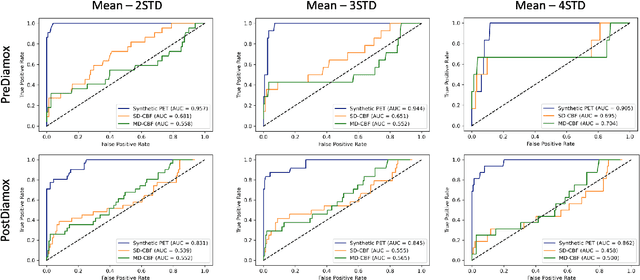
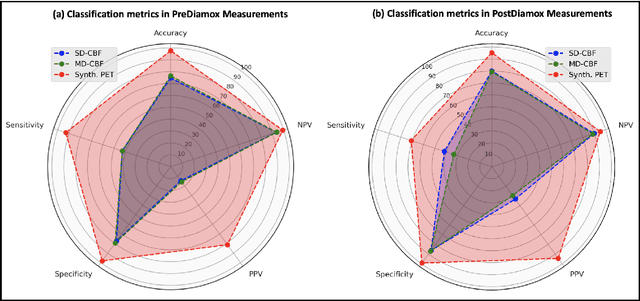
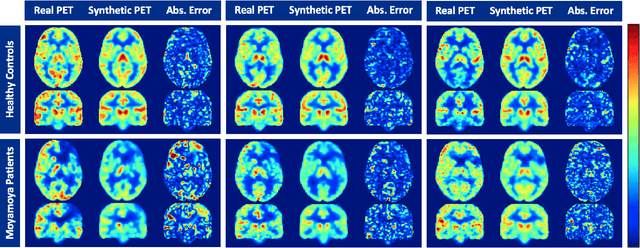
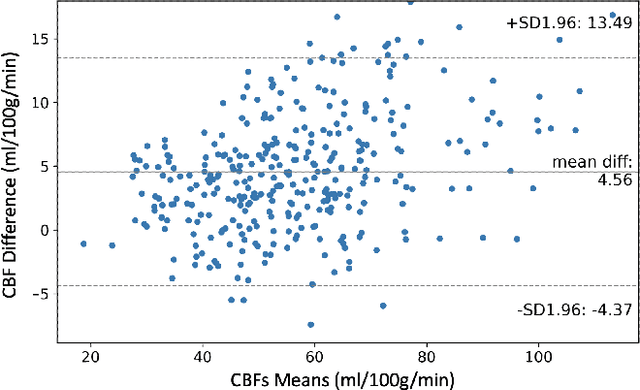
Abstract:Accurate quantification of cerebral blood flow (CBF) is essential for the diagnosis and assessment of a wide range of neurological diseases. Positron emission tomography (PET) with radiolabeled water (15O-water) is considered the gold-standard for the measurement of CBF in humans. PET imaging, however, is not widely available because of its prohibitive costs and use of short-lived radiopharmaceutical tracers that typically require onsite cyclotron production. Magnetic resonance imaging (MRI), in contrast, is more readily accessible and does not involve ionizing radiation. This study presents a convolutional encoder-decoder network with attention mechanisms to predict gold-standard 15O-water PET CBF from multi-sequence MRI scans, thereby eliminating the need for radioactive tracers. Inputs to the prediction model include several commonly used MRI sequences (T1-weighted, T2-FLAIR, and arterial spin labeling). The model was trained and validated using 5-fold cross-validation in a group of 126 subjects consisting of healthy controls and cerebrovascular disease patients, all of whom underwent simultaneous $15O-water PET/MRI. The results show that such a model can successfully synthesize high-quality PET CBF measurements (with an average SSIM of 0.924 and PSNR of 38.8 dB) and is more accurate compared to concurrent and previous PET synthesis methods. We also demonstrate the clinical significance of the proposed algorithm by evaluating the agreement for identifying the vascular territories with abnormally low CBF. Such methods may enable more widespread and accurate CBF evaluation in larger cohorts who cannot undergo PET imaging due to radiation concerns, lack of access, or logistic challenges.
Multi-task Deep Learning for Cerebrovascular Disease Classification and MRI-to-PET Translation
Feb 12, 2022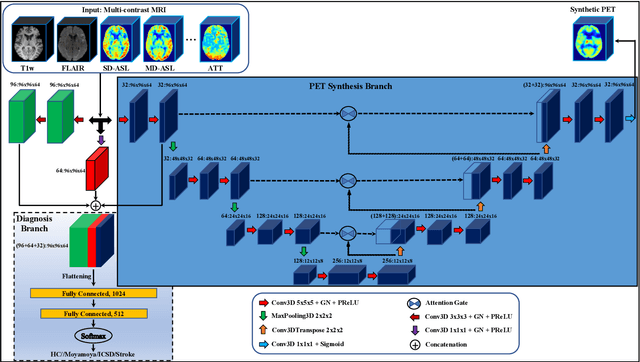
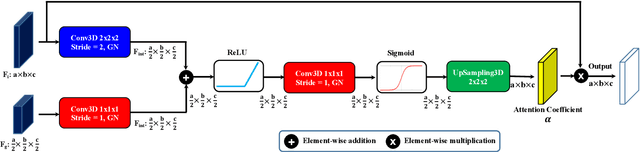
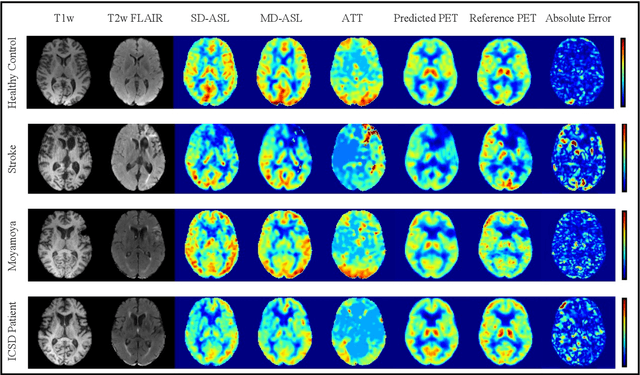
Abstract:Accurate quantification of cerebral blood flow (CBF) is essential for the diagnosis and assessment of cerebrovascular diseases such as Moyamoya, carotid stenosis, aneurysms, and stroke. Positron emission tomography (PET) is currently regarded as the gold standard for the measurement of CBF in the human brain. PET imaging, however, is not widely available because of its prohibitive costs, use of ionizing radiation, and logistical challenges, which require a co-localized cyclotron to deliver the 2 min half-life Oxygen-15 radioisotope. Magnetic resonance imaging (MRI), in contrast, is more readily available and does not involve ionizing radiation. In this study, we propose a multi-task learning framework for brain MRI-to-PET translation and disease diagnosis. The proposed framework comprises two prime networks: (1) an attention-based 3D encoder-decoder convolutional neural network (CNN) that synthesizes high-quality PET CBF maps from multi-contrast MRI images, and (2) a multi-scale 3D CNN that identifies the brain disease corresponding to the input MRI images. Our multi-task framework yields promising results on the task of MRI-to-PET translation, achieving an average structural similarity index (SSIM) of 0.94 and peak signal-to-noise ratio (PSNR) of 38dB on a cohort of 120 subjects. In addition, we show that integrating multiple MRI modalities can improve the clinical diagnosis of brain diseases.
OncoNet: Weakly Supervised Siamese Network to automate cancer treatment response assessment between longitudinal FDG PET/CT examinations
Aug 03, 2021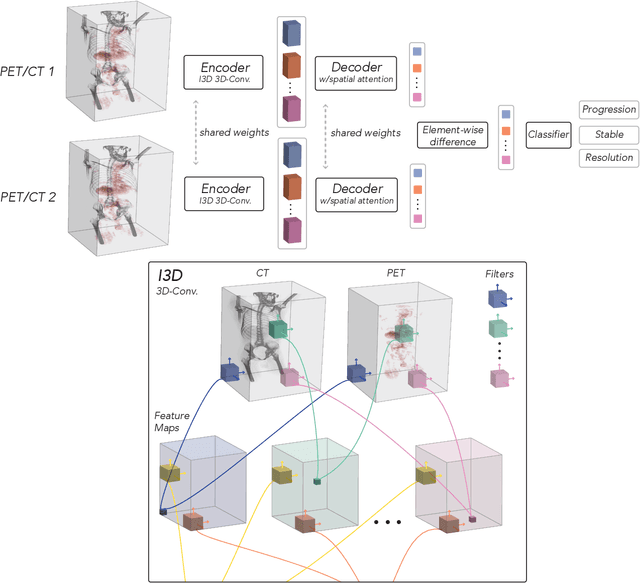

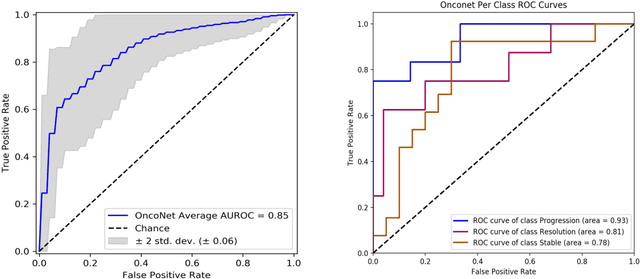
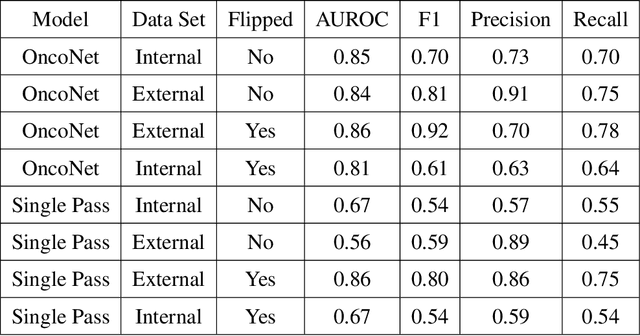
Abstract:FDG PET/CT imaging is a resource intensive examination critical for managing malignant disease and is particularly important for longitudinal assessment during therapy. Approaches to automate longtudinal analysis present many challenges including lack of available longitudinal datasets, managing complex large multimodal imaging examinations, and need for detailed annotations for traditional supervised machine learning. In this work we develop OncoNet, novel machine learning algorithm that assesses treatment response from a 1,954 pairs of sequential FDG PET/CT exams through weak supervision using the standard uptake values (SUVmax) in associated radiology reports. OncoNet demonstrates an AUROC of 0.86 and 0.84 on internal and external institution test sets respectively for determination of change between scans while also showing strong agreement to clinical scoring systems with a kappa score of 0.8. We also curated a dataset of 1,954 paired FDG PET/CT exams designed for response assessment for the broader machine learning in healthcare research community. Automated assessment of radiographic response from FDG PET/CT with OncoNet could provide clinicians with a valuable tool to rapidly and consistently interpret change over time in longitudinal multi-modal imaging exams.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge