Arjun Soin
CheXstray: Real-time Multi-Modal Data Concordance for Drift Detection in Medical Imaging AI
Feb 06, 2022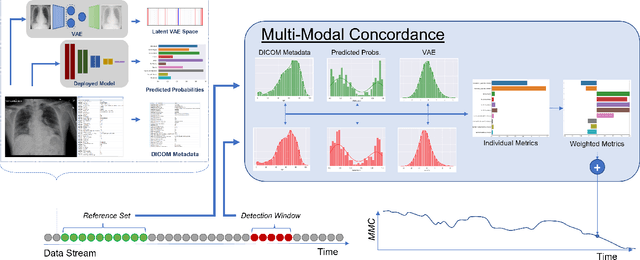
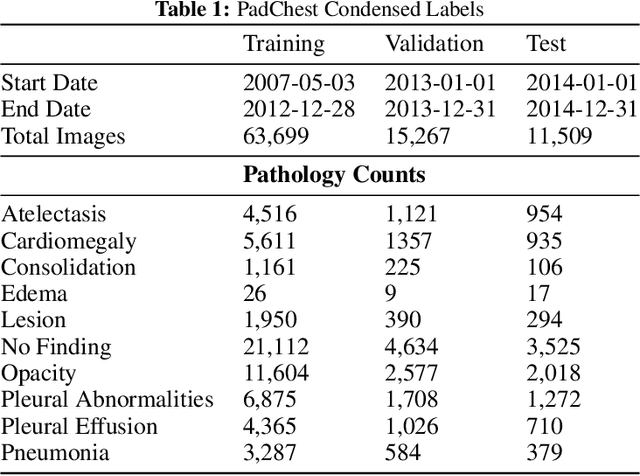
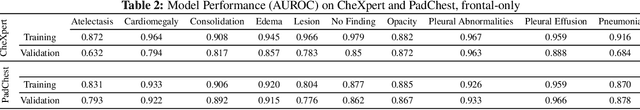
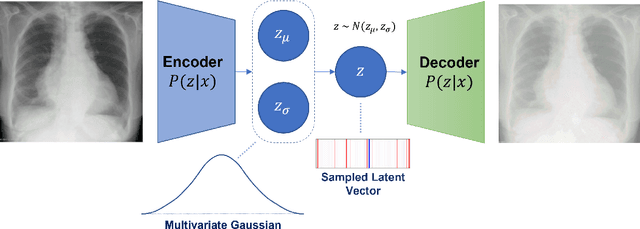
Abstract:Rapidly expanding Clinical AI applications worldwide have the potential to impact to all areas of medical practice. Medical imaging applications constitute a vast majority of approved clinical AI applications. Though healthcare systems are eager to adopt AI solutions a fundamental question remains: \textit{what happens after the AI model goes into production?} We use the CheXpert and PadChest public datasets to build and test a medical imaging AI drift monitoring workflow that tracks data and model drift without contemporaneous ground truth. We simulate drift in multiple experiments to compare model performance with our novel multi-modal drift metric, which uses DICOM metadata, image appearance representation from a variational autoencoder (VAE), and model output probabilities as input. Through experimentation, we demonstrate a strong proxy for ground truth performance using unsupervised distributional shifts in relevant metadata, predicted probabilities, and VAE latent representation. Our key contributions include (1) proof-of-concept for medical imaging drift detection including use of VAE and domain specific statistical methods (2) a multi-modal methodology for measuring and unifying drift metrics (3) new insights into the challenges and solutions for observing deployed medical imaging AI (4) creation of open-source tools enabling others to easily run their own workflows or scenarios. This work has important implications for addressing the translation gap related to continuous medical imaging AI model monitoring in dynamic healthcare environments.
RapidRead: Global Deployment of State-of-the-art Radiology AI for a Large Veterinary Teleradiology Practice
Nov 09, 2021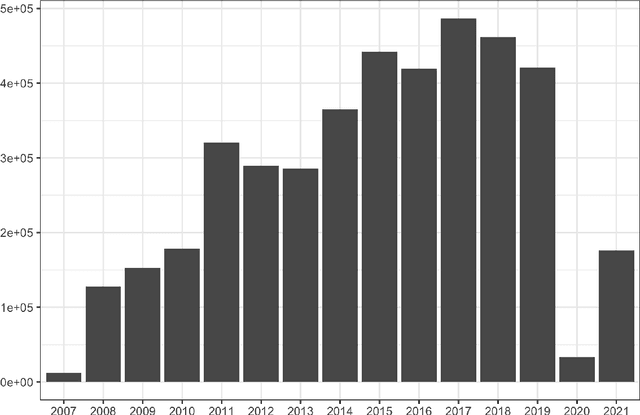
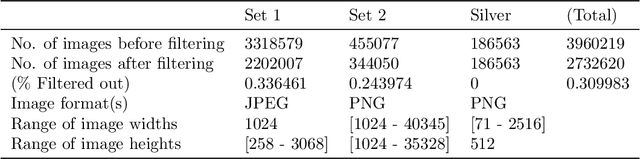
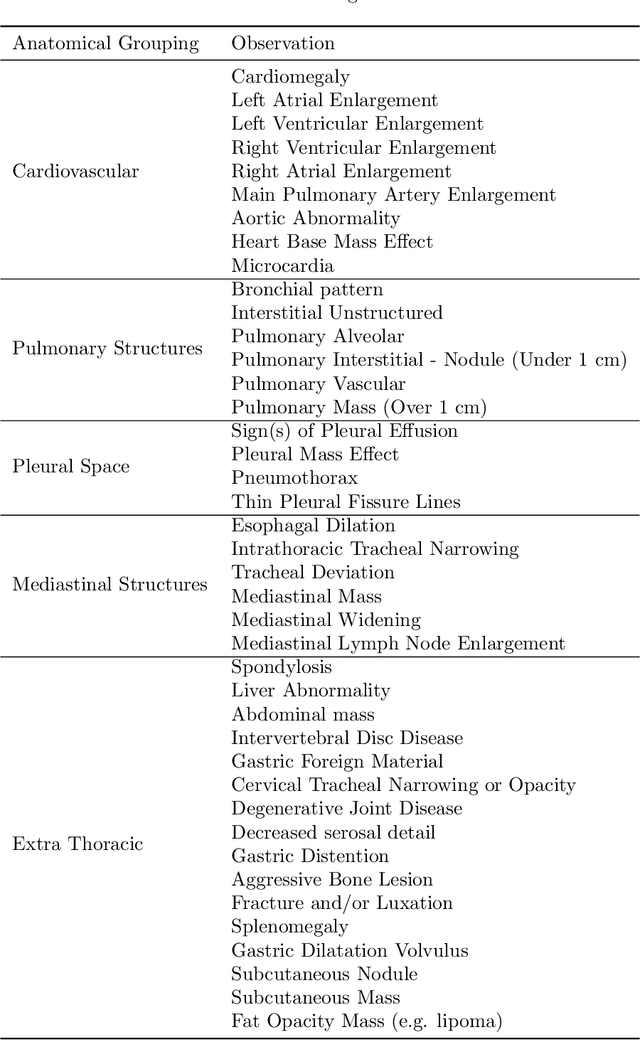
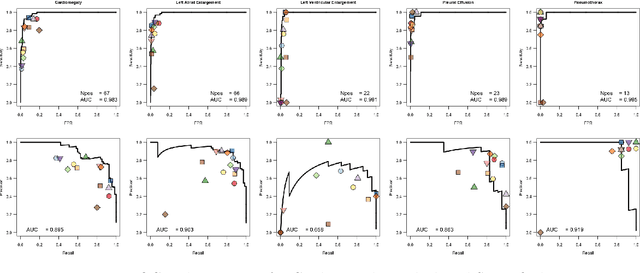
Abstract:This work describes the development and real-world deployment of a deep learning-based AI system for evaluating canine and feline radiographs across a broad range of findings and abnormalities. We describe a new semi-supervised learning approach that combines NLP-derived labels with self-supervised training leveraging more than 2.5 million x-ray images. Finally we describe the clinical deployment of the model including system architecture, real-time performance evaluation and data drift detection.
OncoNet: Weakly Supervised Siamese Network to automate cancer treatment response assessment between longitudinal FDG PET/CT examinations
Aug 03, 2021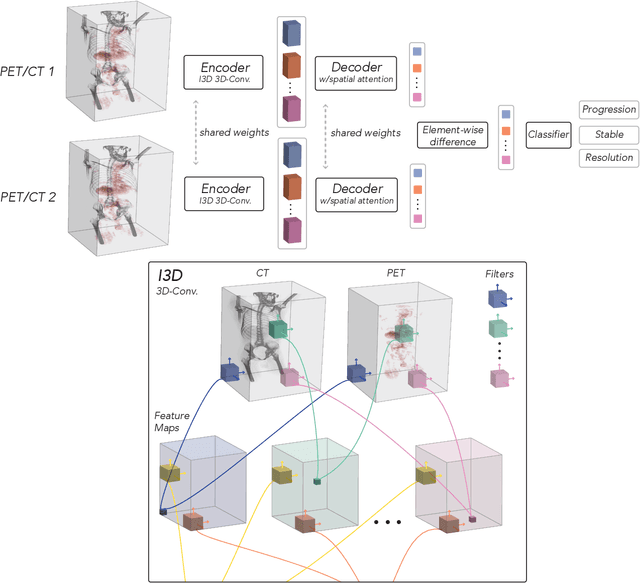

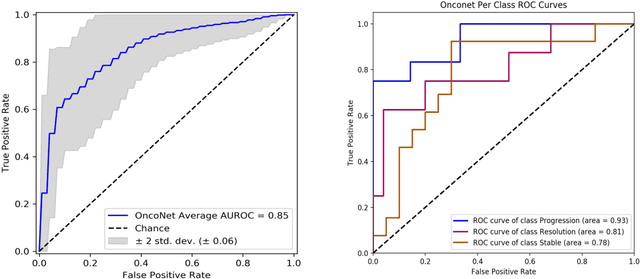
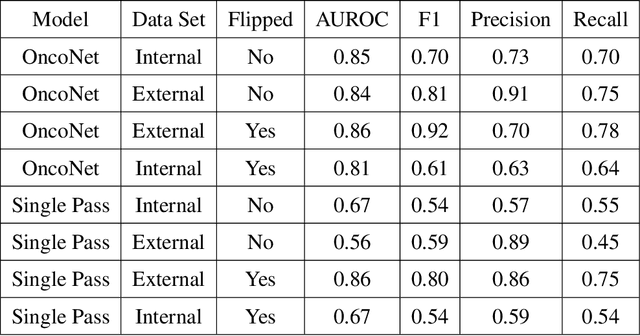
Abstract:FDG PET/CT imaging is a resource intensive examination critical for managing malignant disease and is particularly important for longitudinal assessment during therapy. Approaches to automate longtudinal analysis present many challenges including lack of available longitudinal datasets, managing complex large multimodal imaging examinations, and need for detailed annotations for traditional supervised machine learning. In this work we develop OncoNet, novel machine learning algorithm that assesses treatment response from a 1,954 pairs of sequential FDG PET/CT exams through weak supervision using the standard uptake values (SUVmax) in associated radiology reports. OncoNet demonstrates an AUROC of 0.86 and 0.84 on internal and external institution test sets respectively for determination of change between scans while also showing strong agreement to clinical scoring systems with a kappa score of 0.8. We also curated a dataset of 1,954 paired FDG PET/CT exams designed for response assessment for the broader machine learning in healthcare research community. Automated assessment of radiographic response from FDG PET/CT with OncoNet could provide clinicians with a valuable tool to rapidly and consistently interpret change over time in longitudinal multi-modal imaging exams.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge