Ramy Hussein
Brain MRI-to-PET Synthesis using 3D Convolutional Attention Networks
Nov 22, 2022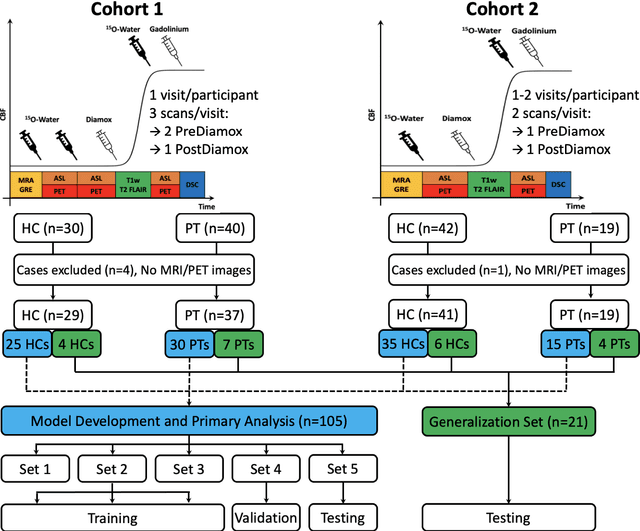
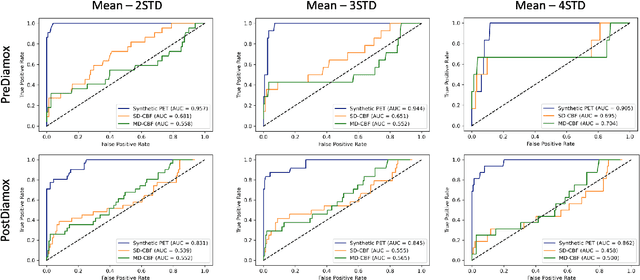
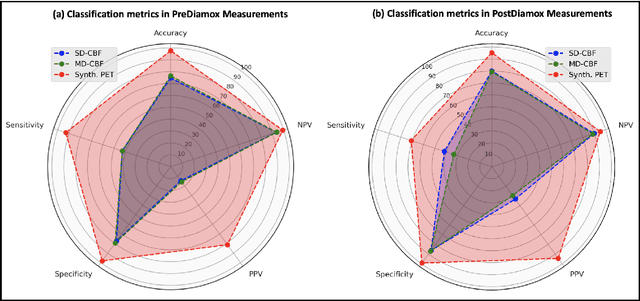
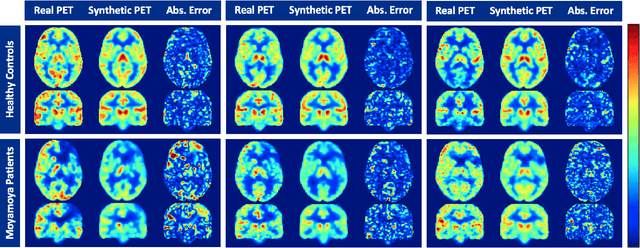
Abstract:Accurate quantification of cerebral blood flow (CBF) is essential for the diagnosis and assessment of a wide range of neurological diseases. Positron emission tomography (PET) with radiolabeled water (15O-water) is considered the gold-standard for the measurement of CBF in humans. PET imaging, however, is not widely available because of its prohibitive costs and use of short-lived radiopharmaceutical tracers that typically require onsite cyclotron production. Magnetic resonance imaging (MRI), in contrast, is more readily accessible and does not involve ionizing radiation. This study presents a convolutional encoder-decoder network with attention mechanisms to predict gold-standard 15O-water PET CBF from multi-sequence MRI scans, thereby eliminating the need for radioactive tracers. Inputs to the prediction model include several commonly used MRI sequences (T1-weighted, T2-FLAIR, and arterial spin labeling). The model was trained and validated using 5-fold cross-validation in a group of 126 subjects consisting of healthy controls and cerebrovascular disease patients, all of whom underwent simultaneous $15O-water PET/MRI. The results show that such a model can successfully synthesize high-quality PET CBF measurements (with an average SSIM of 0.924 and PSNR of 38.8 dB) and is more accurate compared to concurrent and previous PET synthesis methods. We also demonstrate the clinical significance of the proposed algorithm by evaluating the agreement for identifying the vascular territories with abnormally low CBF. Such methods may enable more widespread and accurate CBF evaluation in larger cohorts who cannot undergo PET imaging due to radiation concerns, lack of access, or logistic challenges.
Cascaded Deep Hybrid Models for Multistep Household Energy Consumption Forecasting
Jul 06, 2022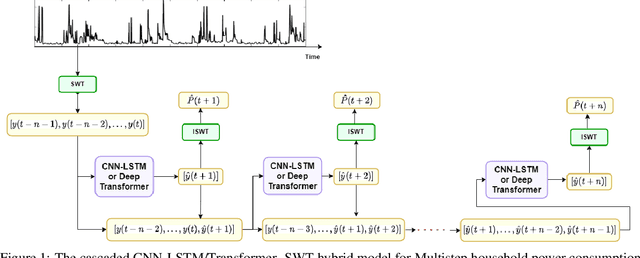

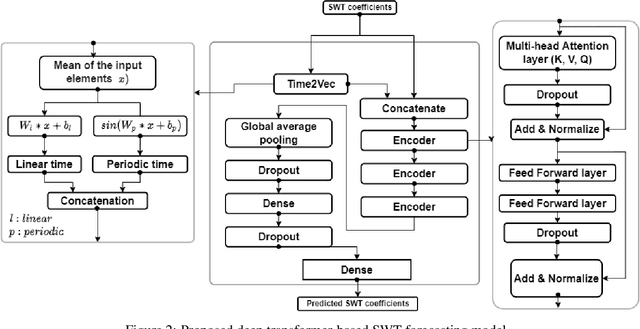

Abstract:Sustainability requires increased energy efficiency with minimal waste. The future power systems should thus provide high levels of flexibility iin controling energy consumption. Precise projections of future energy demand/load at the aggregate and on the individual site levels are of great importance for decision makers and professionals in the energy industry. Forecasting energy loads has become more advantageous for energy providers and customers, allowing them to establish an efficient production strategy to satisfy demand. This study introduces two hybrid cascaded models for forecasting multistep household power consumption in different resolutions. The first model integrates Stationary Wavelet Transform (SWT), as an efficient signal preprocessing technique, with Convolutional Neural Networks and Long Short Term Memory (LSTM). The second hybrid model combines SWT with a self-attention based neural network architecture named transformer. The major constraint of using time-frequency analysis methods such as SWT in multistep energy forecasting problems is that they require sequential signals, making signal reconstruction problematic in multistep forecasting applications.The cascaded models can efficiently address this problem through using the recursive outputs. Experimental results show that the proposed hybrid models achieve superior prediction performance compared to the existing multistep power consumption prediction methods. The results will pave the way for more accurate and reliable forecasting of household power consumption.
Multi-task Deep Learning for Cerebrovascular Disease Classification and MRI-to-PET Translation
Feb 12, 2022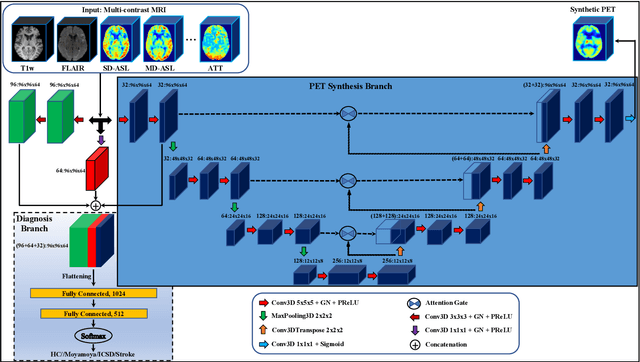
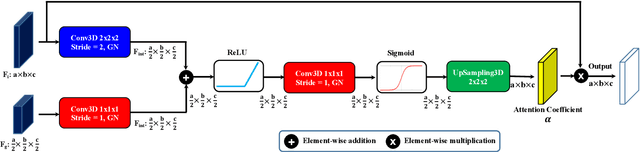
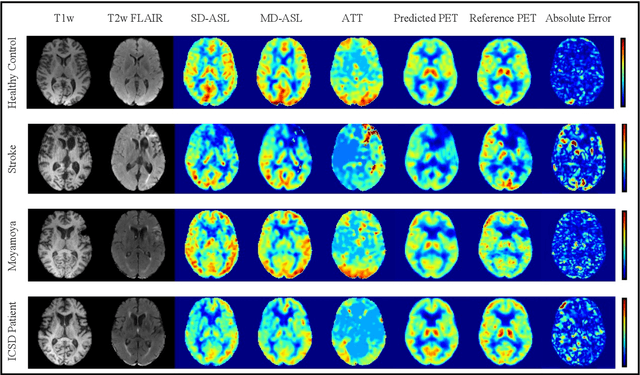
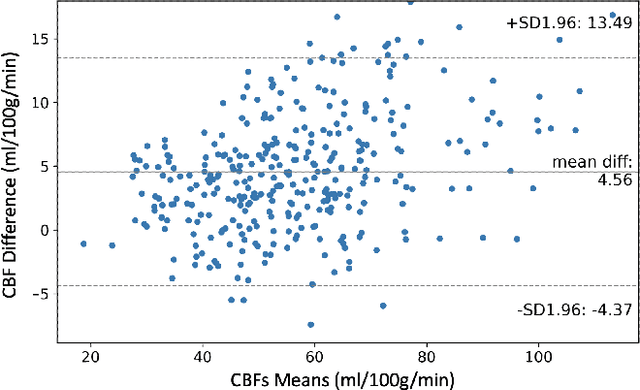
Abstract:Accurate quantification of cerebral blood flow (CBF) is essential for the diagnosis and assessment of cerebrovascular diseases such as Moyamoya, carotid stenosis, aneurysms, and stroke. Positron emission tomography (PET) is currently regarded as the gold standard for the measurement of CBF in the human brain. PET imaging, however, is not widely available because of its prohibitive costs, use of ionizing radiation, and logistical challenges, which require a co-localized cyclotron to deliver the 2 min half-life Oxygen-15 radioisotope. Magnetic resonance imaging (MRI), in contrast, is more readily available and does not involve ionizing radiation. In this study, we propose a multi-task learning framework for brain MRI-to-PET translation and disease diagnosis. The proposed framework comprises two prime networks: (1) an attention-based 3D encoder-decoder convolutional neural network (CNN) that synthesizes high-quality PET CBF maps from multi-contrast MRI images, and (2) a multi-scale 3D CNN that identifies the brain disease corresponding to the input MRI images. Our multi-task framework yields promising results on the task of MRI-to-PET translation, achieving an average structural similarity index (SSIM) of 0.94 and peak signal-to-noise ratio (PSNR) of 38dB on a cohort of 120 subjects. In addition, we show that integrating multiple MRI modalities can improve the clinical diagnosis of brain diseases.
Human Intracranial EEG Quantitative Analysis and Automatic Feature Learning for Epileptic Seizure Prediction
Apr 07, 2019
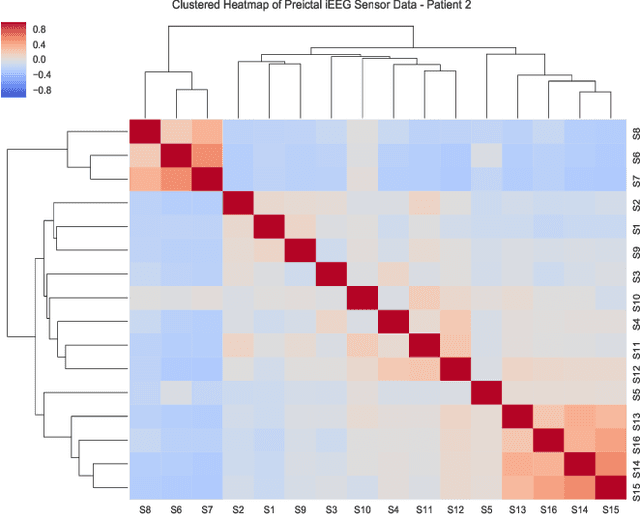
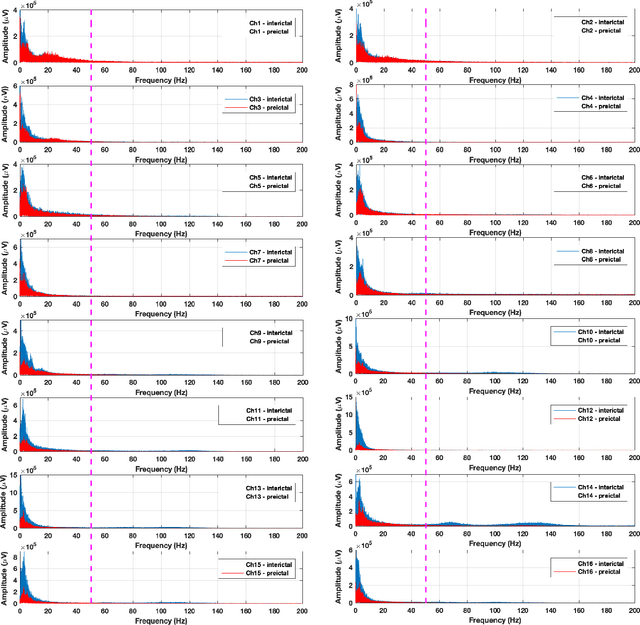
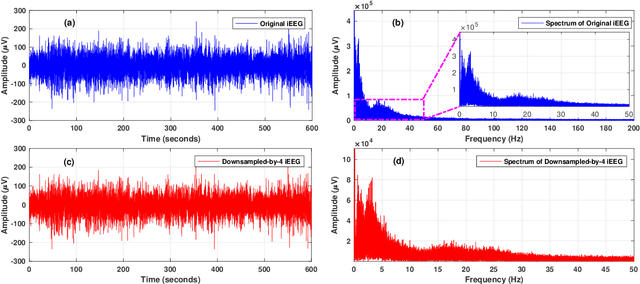
Abstract:Objective: The aim of this study is to develop an efficient and reliable epileptic seizure prediction system using intracranial EEG (iEEG) data, especially for people with drug-resistant epilepsy. The prediction procedure should yield accurate results in a fast enough fashion to alert patients of impending seizures. Methods: We quantitatively analyze the human iEEG data to obtain insights into how the human brain behaves before and between epileptic seizures. We then introduce an efficient pre-processing method for reducing the data size and converting the time-series iEEG data into an image-like format that can be used as inputs to convolutional neural networks (CNNs). Further, we propose a seizure prediction algorithm that uses cooperative multi-scale CNNs for automatic feature learning of iEEG data. Results: 1) iEEG channels contain complementary information and excluding individual channels is not advisable to retain the spatial information needed for accurate prediction of epileptic seizures. 2) The traditional PCA is not a reliable method for iEEG data reduction in seizure prediction. 3) Hand-crafted iEEG features may not be suitable for reliable seizure prediction performance as the iEEG data varies between patients and over time for the same patient. 4) Seizure prediction results show that our algorithm outperforms existing methods by achieving an average sensitivity of 87.85% and AUC score of 0.84. Conclusion: Understanding how the human brain behaves before seizure attacks and far from them facilitates better designs of epileptic seizure predictors. Significance: Accurate seizure prediction algorithms can warn patients about the next seizure attack so they could avoid dangerous activities. Medications could then be administered to abort the impending seizure and minimize the risk of injury.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge