Egon Burian
Longitudinal Self-Supervision for COVID-19 Pathology Quantification
Mar 21, 2022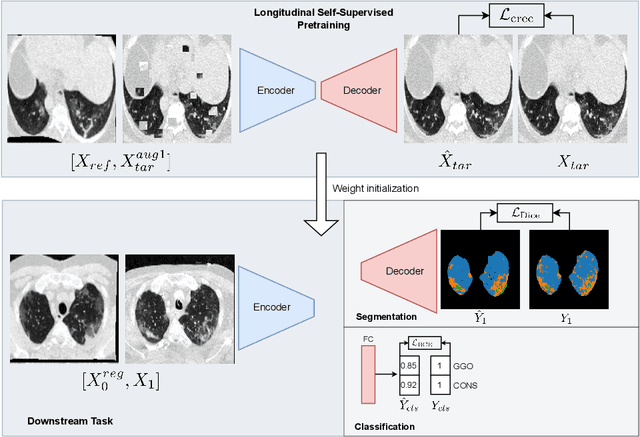
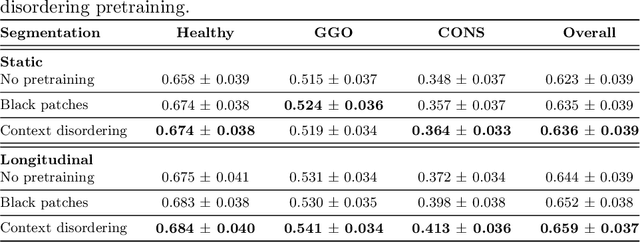
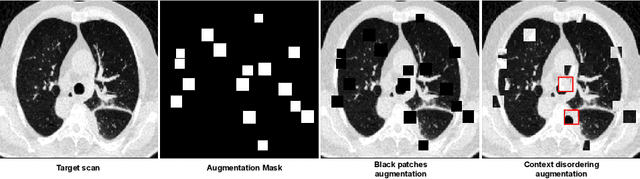

Abstract:Quantifying COVID-19 infection over time is an important task to manage the hospitalization of patients during a global pandemic. Recently, deep learning-based approaches have been proposed to help radiologists automatically quantify COVID-19 pathologies on longitudinal CT scans. However, the learning process of deep learning methods demands extensive training data to learn the complex characteristics of infected regions over longitudinal scans. It is challenging to collect a large-scale dataset, especially for longitudinal training. In this study, we want to address this problem by proposing a new self-supervised learning method to effectively train longitudinal networks for the quantification of COVID-19 infections. For this purpose, longitudinal self-supervision schemes are explored on clinical longitudinal COVID-19 CT scans. Experimental results show that the proposed method is effective, helping the model better exploit the semantics of longitudinal data and improve two COVID-19 quantification tasks.
Interactive Segmentation for COVID-19 Infection Quantification on Longitudinal CT scans
Oct 03, 2021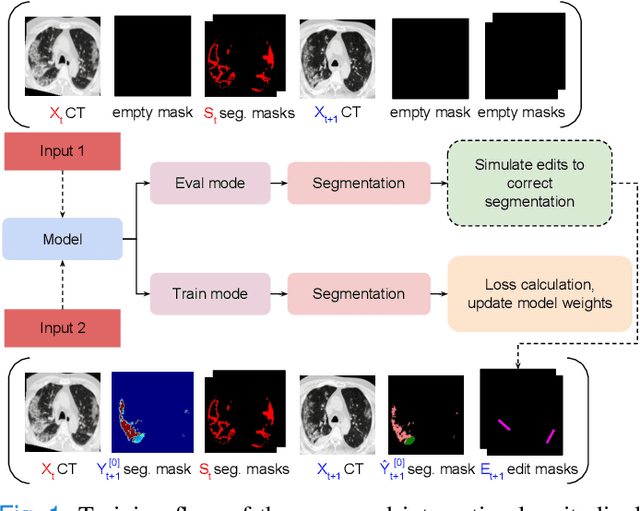
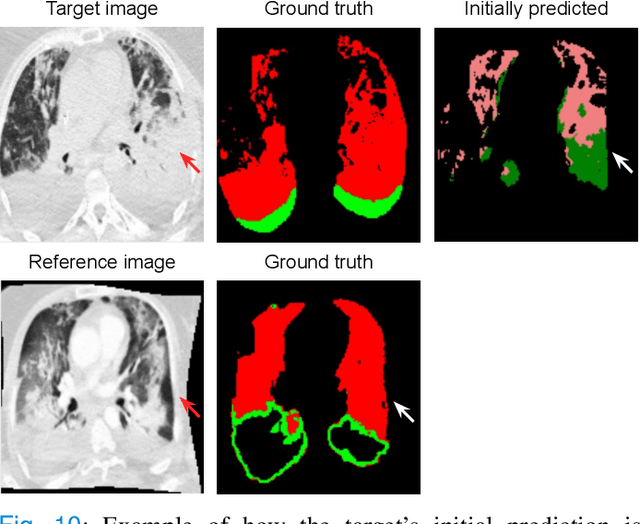
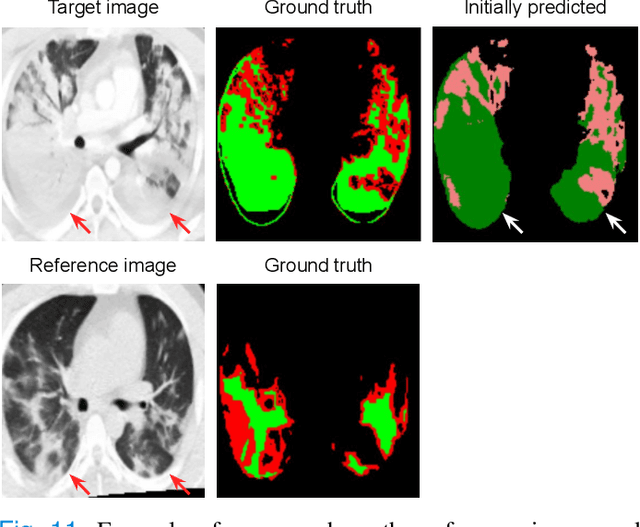
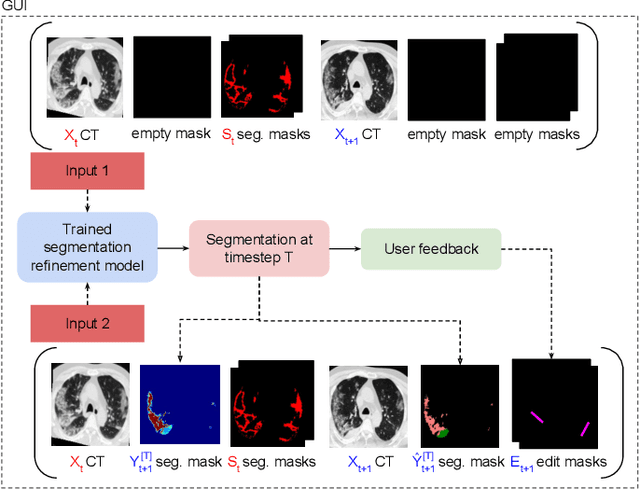
Abstract:Consistent segmentation of COVID-19 patient's CT scans across multiple time points is essential to assess disease progression and response to therapy accurately. Existing automatic and interactive segmentation models for medical images only use data from a single time point (static). However, valuable segmentation information from previous time points is often not used to aid the segmentation of a patient's follow-up scans. Also, fully automatic segmentation techniques frequently produce results that would need further editing for clinical use. In this work, we propose a new single network model for interactive segmentation that fully utilizes all available past information to refine the segmentation of follow-up scans. In the first segmentation round, our model takes 3D volumes of medical images from two-time points (target and reference) as concatenated slices with the additional reference time point segmentation as a guide to segment the target scan. In subsequent segmentation refinement rounds, user feedback in the form of scribbles that correct the segmentation and the target's previous segmentation results are additionally fed into the model. This ensures that the segmentation information from previous refinement rounds is retained. Experimental results on our in-house multiclass longitudinal COVID-19 dataset show that the proposed model outperforms its static version and can assist in localizing COVID-19 infections in patient's follow-up scans.
U-GAT: Multimodal Graph Attention Network for COVID-19 Outcome Prediction
Jul 29, 2021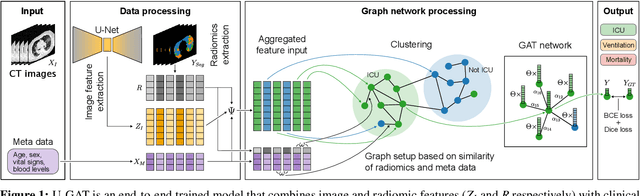
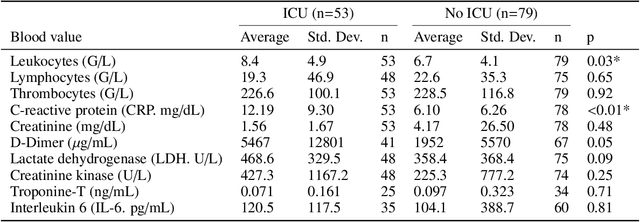
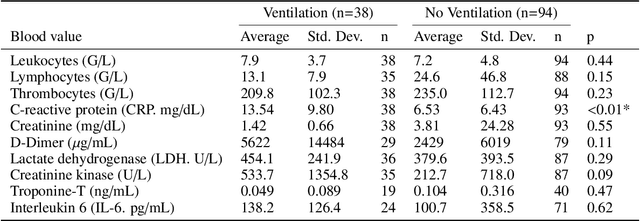
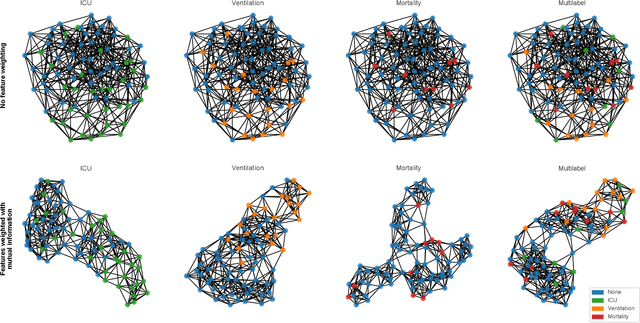
Abstract:During the first wave of COVID-19, hospitals were overwhelmed with the high number of admitted patients. An accurate prediction of the most likely individual disease progression can improve the planning of limited resources and finding the optimal treatment for patients. However, when dealing with a newly emerging disease such as COVID-19, the impact of patient- and disease-specific factors (e.g. body weight or known co-morbidities) on the immediate course of disease is by and large unknown. In the case of COVID-19, the need for intensive care unit (ICU) admission of pneumonia patients is often determined only by acute indicators such as vital signs (e.g. breathing rate, blood oxygen levels), whereas statistical analysis and decision support systems that integrate all of the available data could enable an earlier prognosis. To this end, we propose a holistic graph-based approach combining both imaging and non-imaging information. Specifically, we introduce a multimodal similarity metric to build a population graph for clustering patients and an image-based end-to-end Graph Attention Network to process this graph and predict the COVID-19 patient outcomes: admission to ICU, need for ventilation and mortality. Additionally, the network segments chest CT images as an auxiliary task and extracts image features and radiomics for feature fusion with the available metadata. Results on a dataset collected in Klinikum rechts der Isar in Munich, Germany show that our approach outperforms single modality and non-graph baselines. Moreover, our clustering and graph attention allow for increased understanding of the patient relationships within the population graph and provide insight into the network's decision-making process.
Longitudinal Quantitative Assessment of COVID-19 Infection Progression from Chest CTs
Mar 12, 2021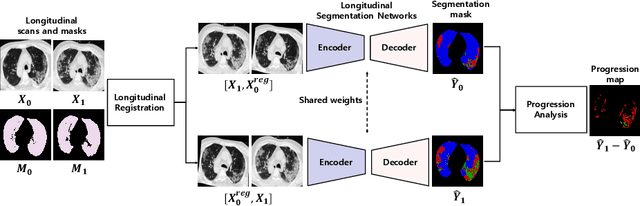
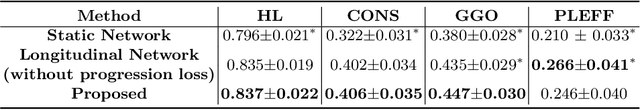

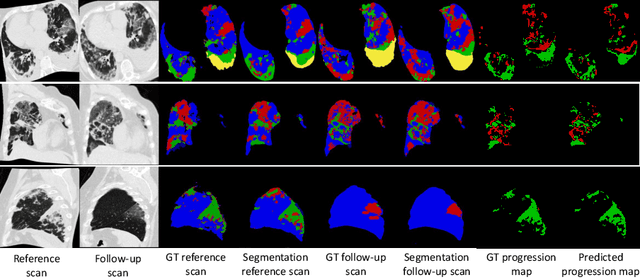
Abstract:Chest computed tomography (CT) has played an essential diagnostic role in assessing patients with COVID-19 by showing disease-specific image features such as ground-glass opacity and consolidation. Image segmentation methods have proven to help quantify the disease burden and even help predict the outcome. The availability of longitudinal CT series may also result in an efficient and effective method to reliably assess the progression of COVID-19, monitor the healing process and the response to different therapeutic strategies. In this paper, we propose a new framework to identify infection at a voxel level (identification of healthy lung, consolidation, and ground-glass opacity) and visualize the progression of COVID-19 using sequential low-dose non-contrast CT scans. In particular, we devise a longitudinal segmentation network that utilizes the reference scan information to improve the performance of disease identification. Experimental results on a clinical longitudinal dataset collected in our institution show the effectiveness of the proposed method compared to the static deep neural networks for disease quantification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge