Chunming Li
Infi-Med: Low-Resource Medical MLLMs with Robust Reasoning Evaluation
May 29, 2025
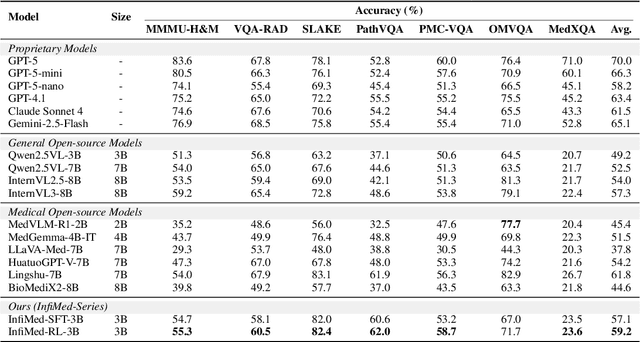
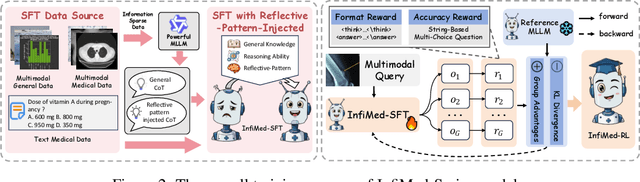
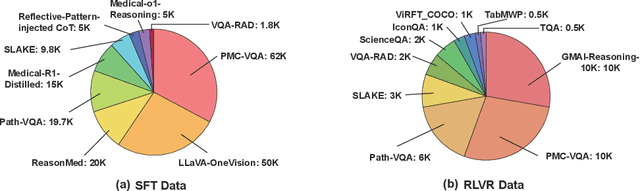
Abstract:Multimodal large language models (MLLMs) have demonstrated promising prospects in healthcare, particularly for addressing complex medical tasks, supporting multidisciplinary treatment (MDT), and enabling personalized precision medicine. However, their practical deployment faces critical challenges in resource efficiency, diagnostic accuracy, clinical considerations, and ethical privacy. To address these limitations, we propose Infi-Med, a comprehensive framework for medical MLLMs that introduces three key innovations: (1) a resource-efficient approach through curating and constructing high-quality supervised fine-tuning (SFT) datasets with minimal sample requirements, with a forward-looking design that extends to both pretraining and posttraining phases; (2) enhanced multimodal reasoning capabilities for cross-modal integration and clinical task understanding; and (3) a systematic evaluation system that assesses model performance across medical modalities and task types. Our experiments demonstrate that Infi-Med achieves state-of-the-art (SOTA) performance in general medical reasoning while maintaining rapid adaptability to clinical scenarios. The framework establishes a solid foundation for deploying MLLMs in real-world healthcare settings by balancing model effectiveness with operational constraints.
Few-shot Novel Category Discovery
May 13, 2025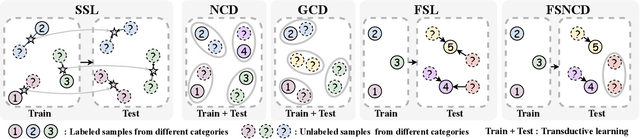
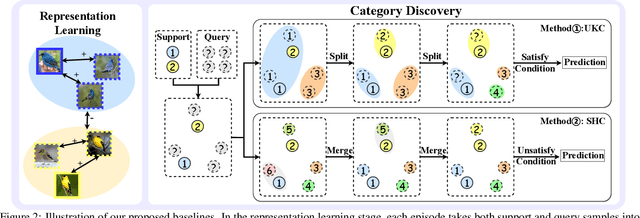
Abstract:The recently proposed Novel Category Discovery (NCD) adapt paradigm of transductive learning hinders its application in more real-world scenarios. In fact, few labeled data in part of new categories can well alleviate this burden, which coincides with the ease that people can label few of new category data. Therefore, this paper presents a new setting in which a trained agent is able to flexibly switch between the tasks of identifying examples of known (labelled) classes and clustering novel (completely unlabeled) classes as the number of query examples increases by leveraging knowledge learned from only a few (handful) support examples. Drawing inspiration from the discovery of novel categories using prior-based clustering algorithms, we introduce a novel framework that further relaxes its assumptions to the real-world open set level by unifying the concept of model adaptability in few-shot learning. We refer to this setting as Few-Shot Novel Category Discovery (FSNCD) and propose Semi-supervised Hierarchical Clustering (SHC) and Uncertainty-aware K-means Clustering (UKC) to examine the model's reasoning capabilities. Extensive experiments and detailed analysis on five commonly used datasets demonstrate that our methods can achieve leading performance levels across different task settings and scenarios.
WSSS4LUAD: Grand Challenge on Weakly-supervised Tissue Semantic Segmentation for Lung Adenocarcinoma
Apr 14, 2022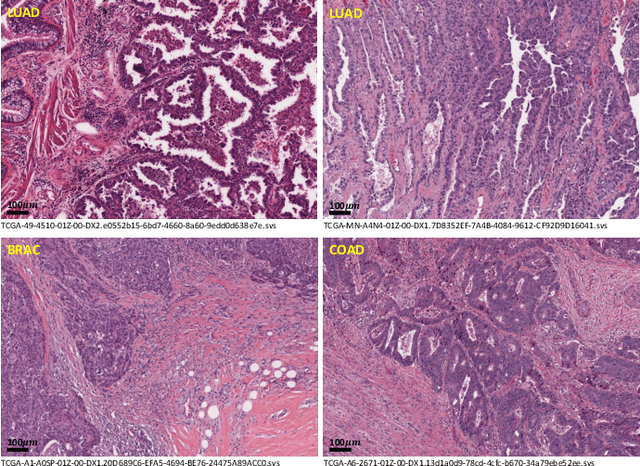
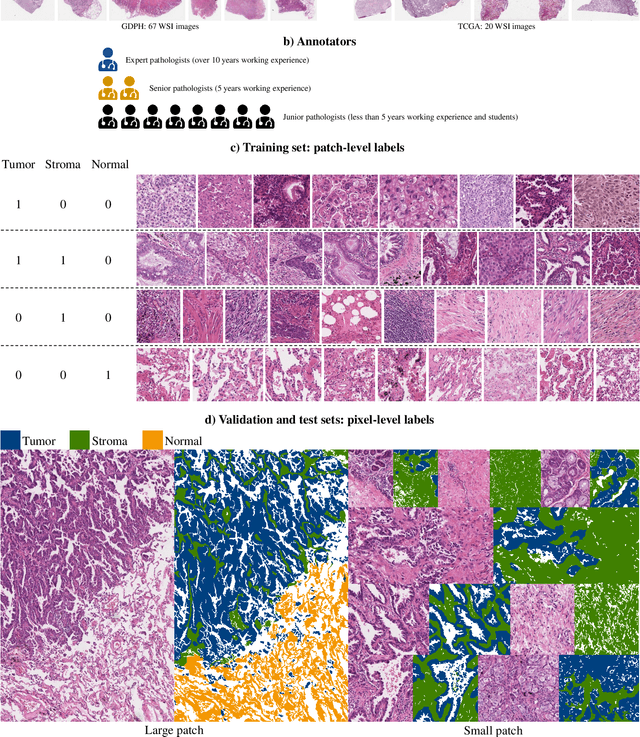
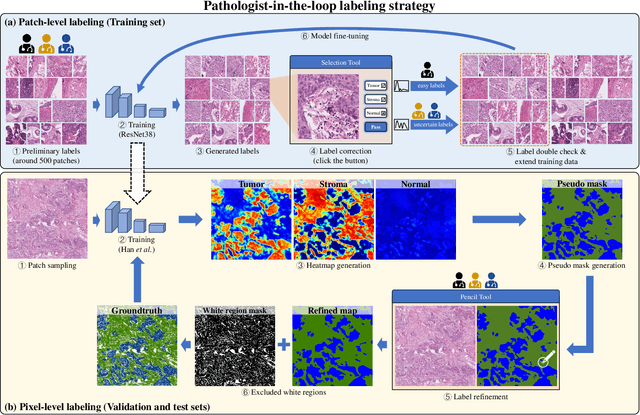
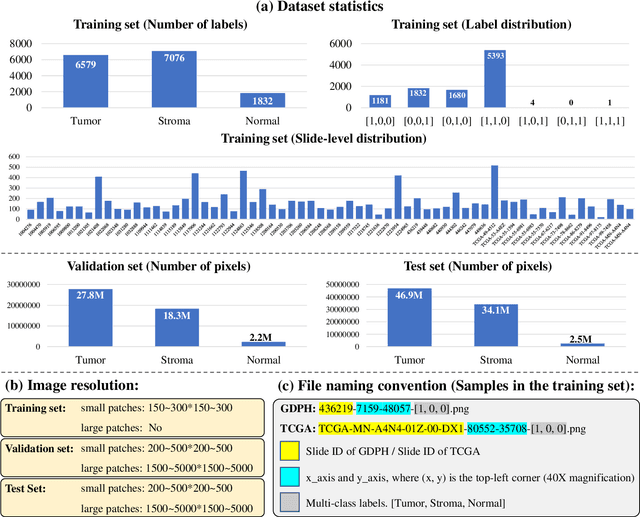
Abstract:Lung cancer is the leading cause of cancer death worldwide, and adenocarcinoma (LUAD) is the most common subtype. Exploiting the potential value of the histopathology images can promote precision medicine in oncology. Tissue segmentation is the basic upstream task of histopathology image analysis. Existing deep learning models have achieved superior segmentation performance but require sufficient pixel-level annotations, which is time-consuming and expensive. To enrich the label resources of LUAD and to alleviate the annotation efforts, we organize this challenge WSSS4LUAD to call for the outstanding weakly-supervised semantic segmentation (WSSS) techniques for histopathology images of LUAD. Participants have to design the algorithm to segment tumor epithelial, tumor-associated stroma and normal tissue with only patch-level labels. This challenge includes 10,091 patch-level annotations (the training set) and over 130 million labeled pixels (the validation and test sets), from 87 WSIs (67 from GDPH, 20 from TCGA). All the labels were generated by a pathologist-in-the-loop pipeline with the help of AI models and checked by the label review board. Among 532 registrations, 28 teams submitted the results in the test phase with over 1,000 submissions. Finally, the first place team achieved mIoU of 0.8413 (tumor: 0.8389, stroma: 0.7931, normal: 0.8919). According to the technical reports of the top-tier teams, CAM is still the most popular approach in WSSS. Cutmix data augmentation has been widely adopted to generate more reliable samples. With the success of this challenge, we believe that WSSS approaches with patch-level annotations can be a complement to the traditional pixel annotations while reducing the annotation efforts. The entire dataset has been released to encourage more researches on computational pathology in LUAD and more novel WSSS techniques.
The Liver Tumor Segmentation Benchmark (LiTS)
Jan 13, 2019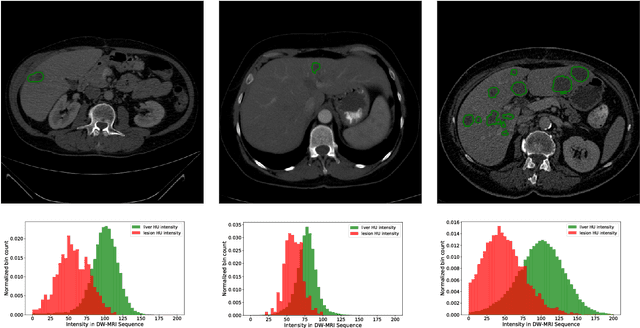

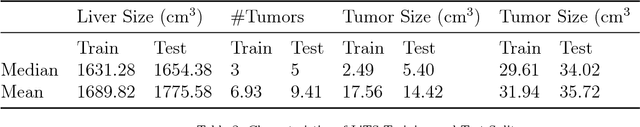
Abstract:In this work, we report the set-up and results of the Liver Tumor Segmentation Benchmark (LITS) organized in conjunction with the IEEE International Symposium on Biomedical Imaging (ISBI) 2016 and International Conference On Medical Image Computing Computer Assisted Intervention (MICCAI) 2017. Twenty four valid state-of-the-art liver and liver tumor segmentation algorithms were applied to a set of 131 computed tomography (CT) volumes with different types of tumor contrast levels (hyper-/hypo-intense), abnormalities in tissues (metastasectomie) size and varying amount of lesions. The submitted algorithms have been tested on 70 undisclosed volumes. The dataset is created in collaboration with seven hospitals and research institutions and manually reviewed by independent three radiologists. We found that not a single algorithm performed best for liver and tumors. The best liver segmentation algorithm achieved a Dice score of 0.96(MICCAI) whereas for tumor segmentation the best algorithm evaluated at 0.67(ISBI) and 0.70(MICCAI). The LITS image data and manual annotations continue to be publicly available through an online evaluation system as an ongoing benchmarking resource.
Global and Local Information Based Deep Network for Skin Lesion Segmentation
Mar 16, 2017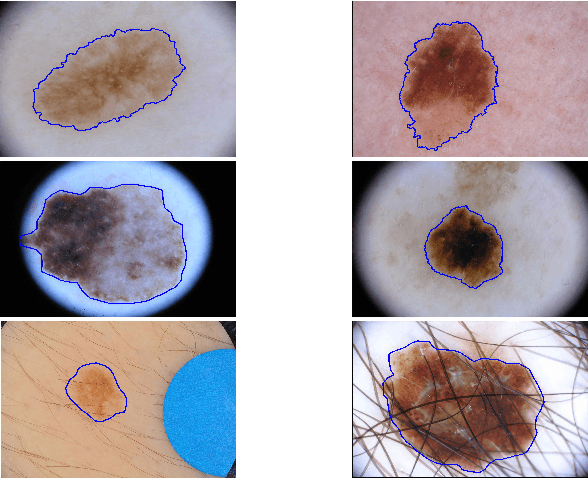
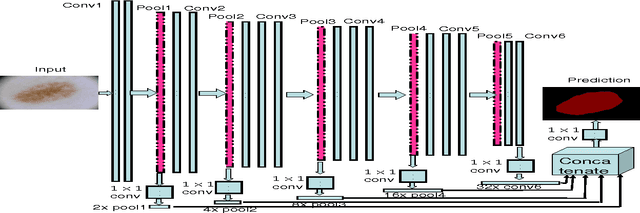
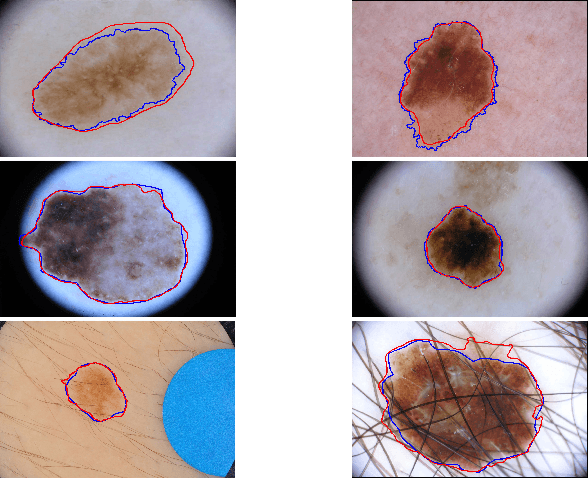
Abstract:With a large influx of dermoscopy images and a growing shortage of dermatologists, automatic dermoscopic image analysis plays an essential role in skin cancer diagnosis. In this paper, a new deep fully convolutional neural network (FCNN) is proposed to automatically segment melanoma out of skin images by end-to-end learning with only pixels and labels as inputs. Our proposed FCNN is capable of using both local and global information to segment melanoma by adopting skipping layers. The public benchmark database consisting of 150 validation images, 600 test images and 2000 training images in the melanoma detection challenge 2017 at International Symposium Biomedical Imaging 2017 is used to test the performance of our algorithm. All large size images (for example, $4000\times 6000$ pixels) are reduced to much smaller images with $384\times 384$ pixels (more than 10 times smaller). We got and submitted preliminary results to the challenge without any pre or post processing. The performance of our proposed method could be further improved by data augmentation and by avoiding image size reduction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge