Charles Huang
Learning Treatment Plan Representations for Content Based Image Retrieval
Jun 06, 2022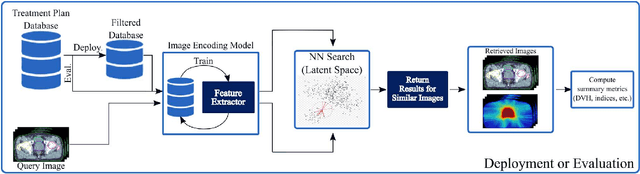
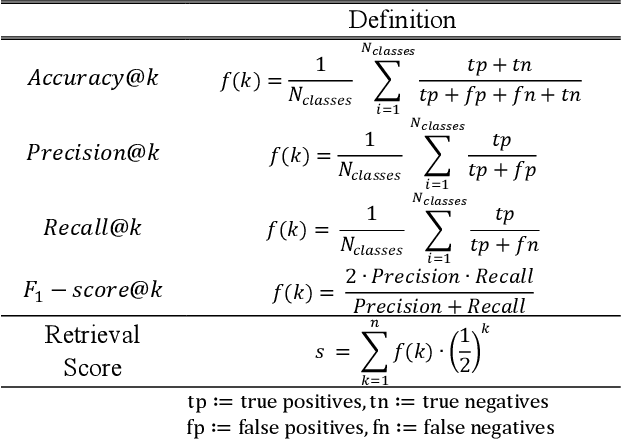
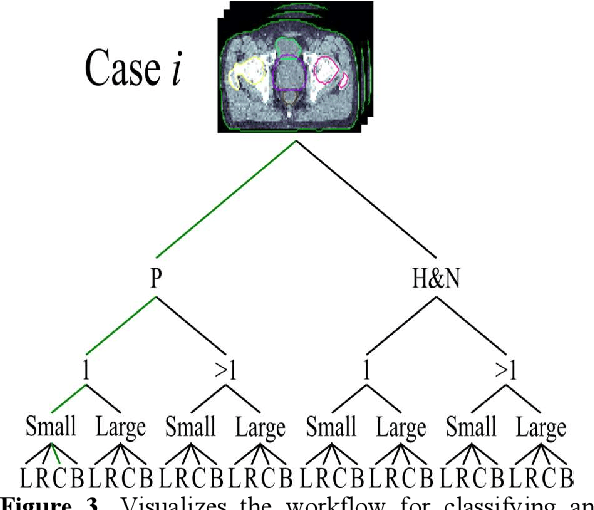
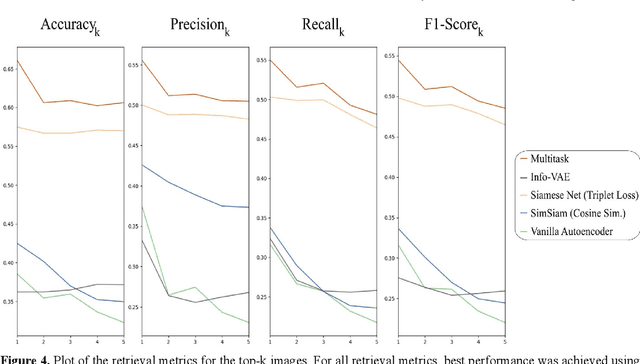
Abstract:Objective: Knowledge based planning (KBP) typically involves training an end-to-end deep learning model to predict dose distributions. However, training end-to-end KBP methods may be associated with practical limitations due to the limited size of medical datasets that are often used. To address these limitations, we propose a content based image retrieval (CBIR) method for retrieving dose distributions of previously planned patients based on anatomical similarity. Approach: Our proposed CBIR method trains a representation model that produces latent space embeddings of a patient's anatomical information. The latent space embeddings of new patients are then compared against those of previous patients in a database for image retrieval of dose distributions. Summary metrics (e.g. dose-volume histogram, conformity index, homogeneity index, etc.) are computed and can then be utilized in subsequent automated planning. All source code for this project is available on github. Main Results: The retrieval performance of various CBIR methods is evaluated on a dataset consisting of both publicly available plans and clinical plans from our institution. This study compares various encoding methods, ranging from simple autoencoders to more recent Siamese networks like SimSiam, and the best performance was observed for the multitask Siamese network. Significance: Applying CBIR to inform subsequent treatment planning potentially addresses many limitations associated with end-to-end KBP. Our current results demonstrate that excellent image retrieval performance can be obtained through slight changes to previously developed Siamese networks. We hope to integrate CBIR into automated planning workflow in future works, potentially through methods like the MetaPlanner framework.
Atlas Based Segmentations via Semi-Supervised Diffeomorphic Registrations
Nov 23, 2019



Abstract:Purpose: Segmentation of organs-at-risk (OARs) is a bottleneck in current radiation oncology pipelines and is often time consuming and labor intensive. In this paper, we propose an atlas-based semi-supervised registration algorithm to generate accurate segmentations of OARs for which there are ground truth contours and rough segmentations of all other OARs in the atlas. To the best of our knowledge, this is the first study to use learning-based registration methods for the segmentation of head and neck patients and demonstrate its utility in clinical applications. Methods: Our algorithm cascades rigid and deformable deformation blocks, and takes on an atlas image (M), set of atlas-space segmentations (S_A), and a patient image (F) as inputs, while outputting patient-space segmentations of all OARs defined on the atlas. We train our model on 475 CT images taken from public archives and Stanford RadOnc Clinic (SROC), validate on 5 CT images from SROC, and test our model on 20 CT images from SROC. Results: Our method outperforms current state of the art learning-based registration algorithms and achieves an overall dice score of 0.789 on our test set. Moreover, our method yields a performance comparable to manual segmentation and supervised segmentation, while solving a much more complex registration problem. Whereas supervised segmentation methods only automate the segmentation process for a select few number of OARs, we demonstrate that our methods can achieve similar performance for OARs of interest, while also providing segmentations for every other OAR on the provided atlas. Conclusions: Our proposed algorithm has significant clinical applications and could help reduce the bottleneck for segmentation of head and neck OARs. Further, our results demonstrate that semi-supervised diffeomorphic registration can be accurately applied to both registration and segmentation problems.
Machine Learning Techniques for Biomedical Image Segmentation: An Overview of Technical Aspects and Introduction to State-of-Art Applications
Nov 06, 2019Abstract:In recent years, significant progress has been made in developing more accurate and efficient machine learning algorithms for segmentation of medical and natural images. In this review article, we highlight the imperative role of machine learning algorithms in enabling efficient and accurate segmentation in the field of medical imaging. We specifically focus on several key studies pertaining to the application of machine learning methods to biomedical image segmentation. We review classical machine learning algorithms such as Markov random fields, k-means clustering, random forest, etc. Although such classical learning models are often less accurate compared to the deep learning techniques, they are often more sample efficient and have a less complex structure. We also review different deep learning architectures, such as the artificial neural networks (ANNs), the convolutional neural networks (CNNs), and the recurrent neural networks (RNNs), and present the segmentation results attained by those learning models that were published in the past three years. We highlight the successes and limitations of each machine learning paradigm. In addition, we discuss several challenges related to the training of different machine learning models, and we present some heuristics to address those challenges.
Modified U-Net with Incorporation of Object-Dependent High Level Features for Improved Liver and Liver-Tumor Segmentation in CT Images
Oct 31, 2019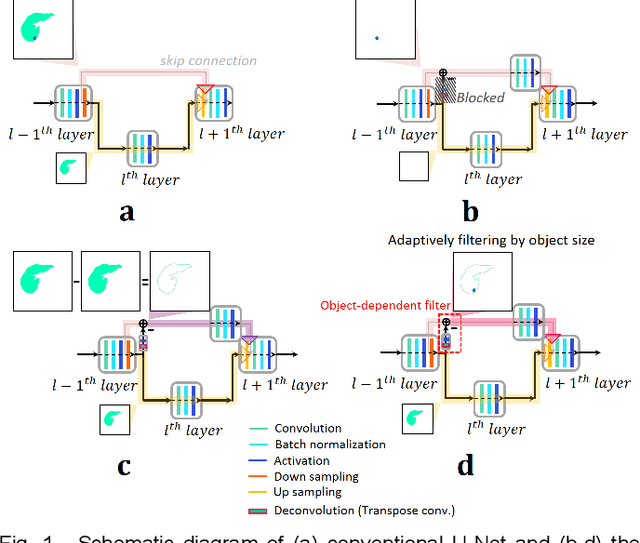
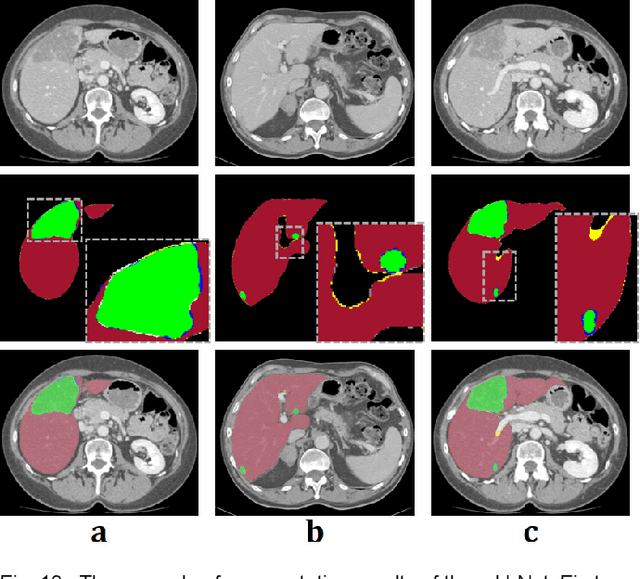
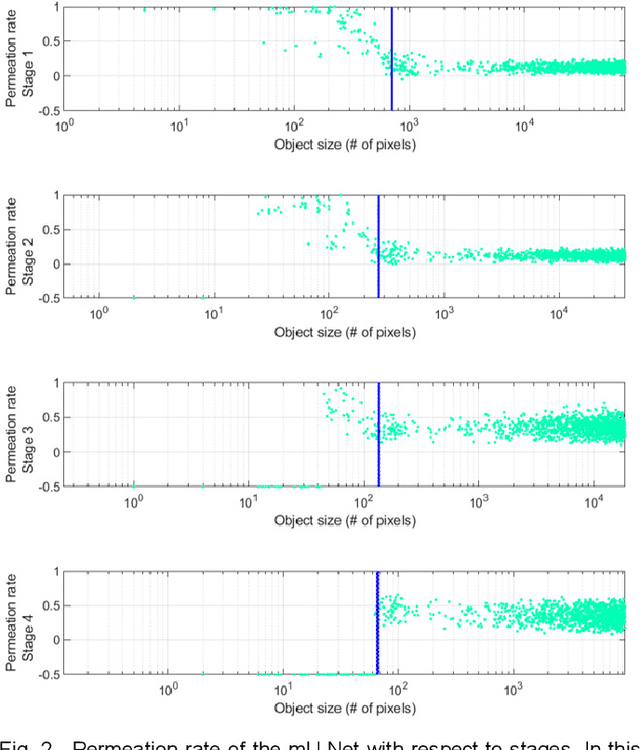

Abstract:Segmentation of livers and liver tumors is one of the most important steps in radiation therapy of hepatocellular carcinoma. The segmentation task is often done manually, making it tedious, labor intensive, and subject to intra-/inter- operator variations. While various algorithms for delineating organ-at-risks (OARs) and tumor targets have been proposed, automatic segmentation of livers and liver tumors remains intractable due to their low tissue contrast with respect to the surrounding organs and their deformable shape in CT images. The U-Net has gained increasing popularity recently for image analysis tasks and has shown promising results. Conventional U-Net architectures, however, suffer from three major drawbacks. To cope with these problems, we added a residual path with deconvolution and activation operations to the skip connection of the U-Net to avoid duplication of low resolution information of features. In the case of small object inputs, features in the skip connection are not incorporated with features in the residual path. Furthermore, the proposed architecture has additional convolution layers in the skip connection in order to extract high level global features of small object inputs as well as high level features of high resolution edge information of large object inputs. Efficacy of the modified U-Net (mU-Net) was demonstrated using the public dataset of Liver tumor segmentation (LiTS) challenge 2017. The proposed mU-Net outperformed existing state-of-art networks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge