Bingchuan Wang
End-to-end cell recognition by point annotation
Jul 01, 2022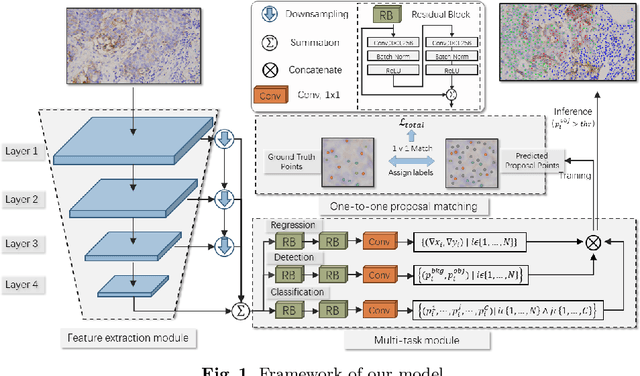
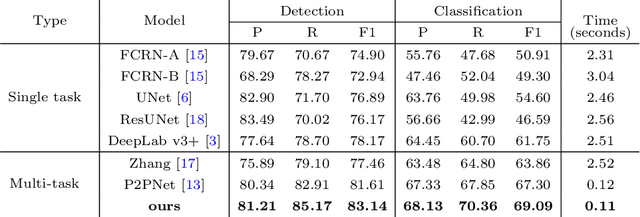
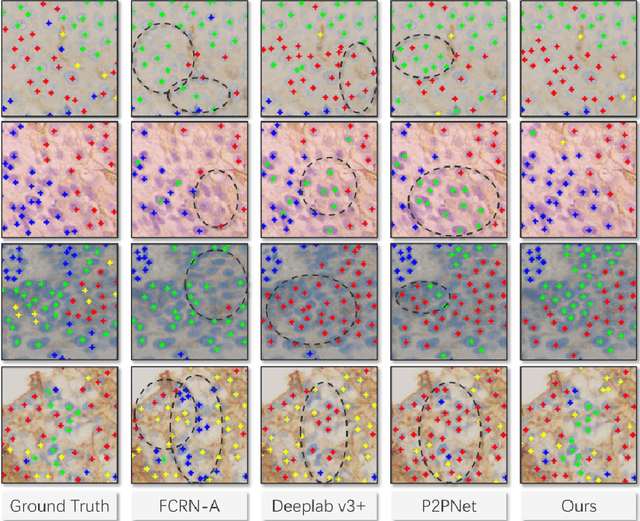
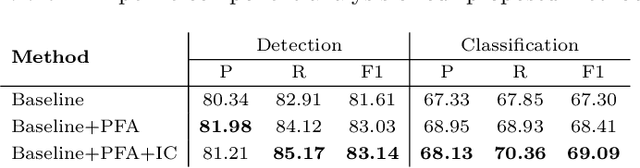
Abstract:Reliable quantitative analysis of immunohistochemical staining images requires accurate and robust cell detection and classification. Recent weakly-supervised methods usually estimate probability density maps for cell recognition. However, in dense cell scenarios, their performance can be limited by pre- and post-processing as it is impossible to find a universal parameter setting. In this paper, we introduce an end-to-end framework that applies direct regression and classification for preset anchor points. Specifically, we propose a pyramidal feature aggregation strategy to combine low-level features and high-level semantics simultaneously, which provides accurate cell recognition for our purely point-based model. In addition, an optimized cost function is designed to adapt our multi-task learning framework by matching ground truth and predicted points. The experimental results demonstrate the superior accuracy and efficiency of the proposed method, which reveals the high potentiality in assisting pathologist assessments.
A Prior Guided Adversarial Representation Learning and Hypergraph Perceptual Network for Predicting Abnormal Connections of Alzheimer's Disease
Oct 12, 2021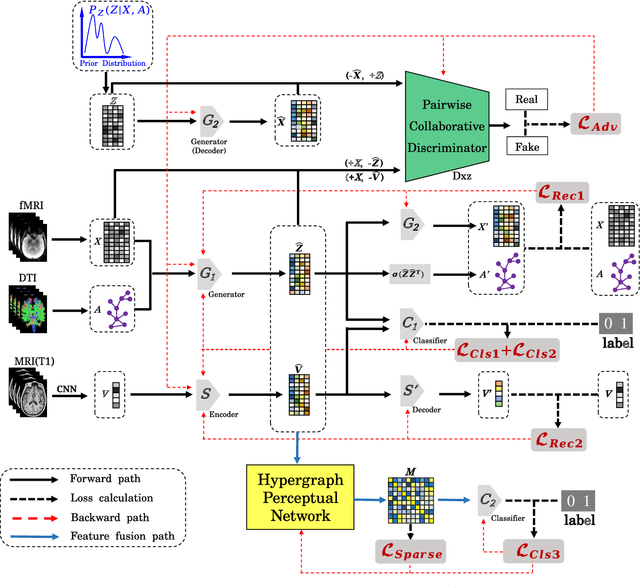
Abstract:Alzheimer's disease is characterized by alterations of the brain's structural and functional connectivity during its progressive degenerative processes. Existing auxiliary diagnostic methods have accomplished the classification task, but few of them can accurately evaluate the changing characteristics of brain connectivity. In this work, a prior guided adversarial representation learning and hypergraph perceptual network (PGARL-HPN) is proposed to predict abnormal brain connections using triple-modality medical images. Concretely, a prior distribution from the anatomical knowledge is estimated to guide multimodal representation learning using an adversarial strategy. Also, the pairwise collaborative discriminator structure is further utilized to narrow the difference of representation distribution. Moreover, the hypergraph perceptual network is developed to effectively fuse the learned representations while establishing high-order relations within and between multimodal images. Experimental results demonstrate that the proposed model outperforms other related methods in analyzing and predicting Alzheimer's disease progression. More importantly, the identified abnormal connections are partly consistent with the previous neuroscience discoveries. The proposed model can evaluate characteristics of abnormal brain connections at different stages of Alzheimer's disease, which is helpful for cognitive disease study and early treatment.
DecGAN: Decoupling Generative Adversarial Network detecting abnormal neural circuits for Alzheimer's disease
Oct 12, 2021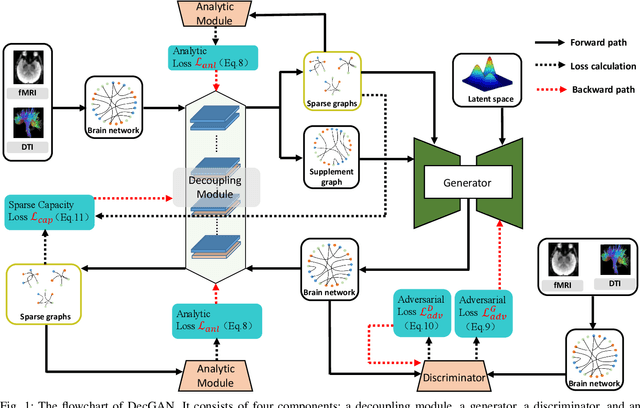
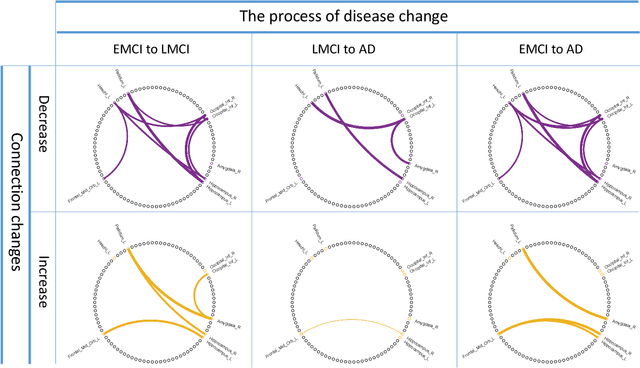
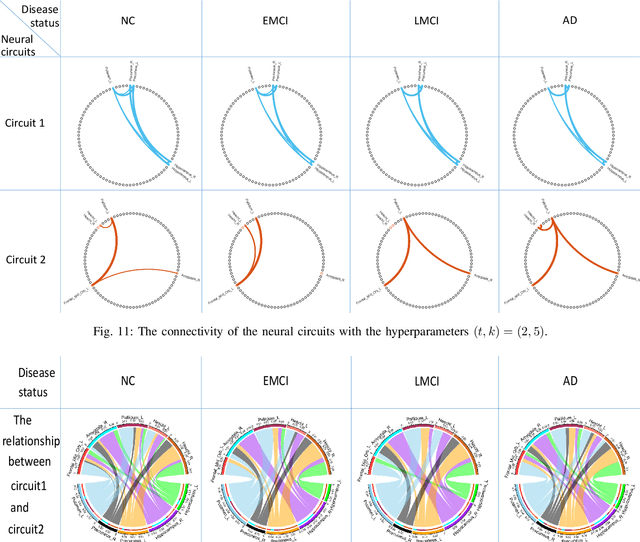
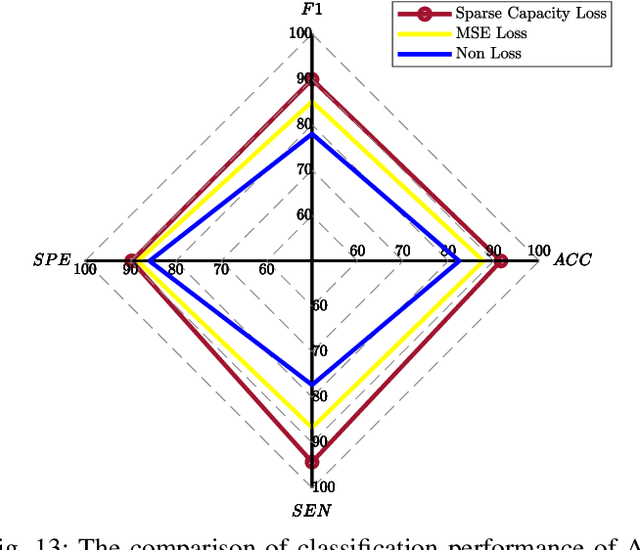
Abstract:One of the main reasons for Alzheimer's disease (AD) is the disorder of some neural circuits. Existing methods for AD prediction have achieved great success, however, detecting abnormal neural circuits from the perspective of brain networks is still a big challenge. In this work, a novel decoupling generative adversarial network (DecGAN) is proposed to detect abnormal neural circuits for AD. Concretely, a decoupling module is designed to decompose a brain network into two parts: one part is composed of a few sparse graphs which represent the neural circuits largely determining the development of AD; the other part is a supplement graph, whose influence on AD can be ignored. Furthermore, the adversarial strategy is utilized to guide the decoupling module to extract the feature more related to AD. Meanwhile, by encoding the detected neural circuits to hypergraph data, an analytic module associated with the hyperedge neurons algorithm is designed to identify the neural circuits. More importantly, a novel sparse capacity loss based on the spatial-spectral hypergraph similarity is developed to minimize the intrinsic topological distribution of neural circuits, which can significantly improve the accuracy and robustness of the proposed model. Experimental results demonstrate that the proposed model can effectively detect the abnormal neural circuits at different stages of AD, which is helpful for pathological study and early treatment.
3D Brain Reconstruction by Hierarchical Shape-Perception Network from a Single Incomplete Image
Jul 23, 2021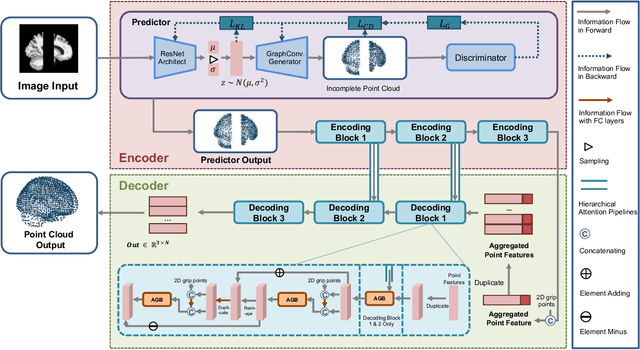
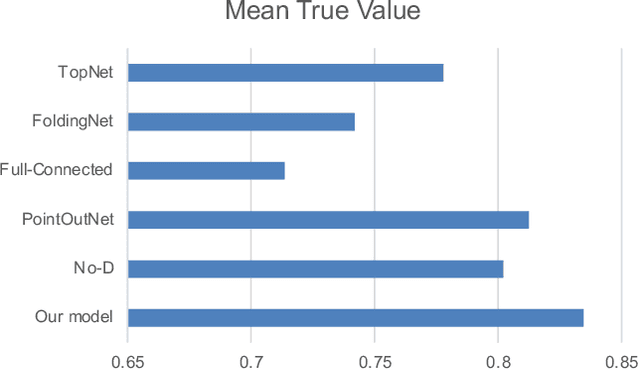
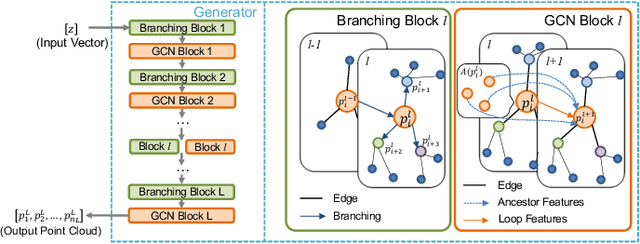
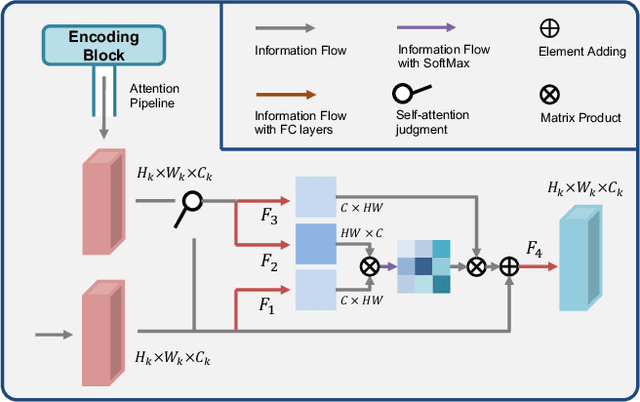
Abstract:3D shape reconstruction is essential in the navigation of minimally-invasive and auto robot-guided surgeries whose operating environments are indirect and narrow, and there have been some works that focused on reconstructing the 3D shape of the surgical organ through limited 2D information available. However, the lack and incompleteness of such information caused by intraoperative emergencies (such as bleeding) and risk control conditions have not been considered. In this paper, a novel hierarchical shape-perception network (HSPN) is proposed to reconstruct the 3D point clouds (PCs) of specific brains from one single incomplete image with low latency. A tree-structured predictor and several hierarchical attention pipelines are constructed to generate point clouds that accurately describe the incomplete images and then complete these point clouds with high quality. Meanwhile, attention gate blocks (AGBs) are designed to efficiently aggregate geometric local features of incomplete PCs transmitted by hierarchical attention pipelines and internal features of reconstructing point clouds. With the proposed HSPN, 3D shape perception and completion can be achieved spontaneously. Comprehensive results measured by Chamfer distance and PC-to-PC error demonstrate that the performance of the proposed HSPN outperforms other competitive methods in terms of qualitative displays, quantitative experiment, and classification evaluation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge