Junren Pan
Alzheimer's Disease Prediction via Brain Structural-Functional Deep Fusing Network
Oct 05, 2023Abstract:Fusing structural-functional images of the brain has shown great potential to analyze the deterioration of Alzheimer's disease (AD). However, it is a big challenge to effectively fuse the correlated and complementary information from multimodal neuroimages. In this paper, a novel model termed cross-modal transformer generative adversarial network (CT-GAN) is proposed to effectively fuse the functional and structural information contained in functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI). The CT-GAN can learn topological features and generate multimodal connectivity from multimodal imaging data in an efficient end-to-end manner. Moreover, the swapping bi-attention mechanism is designed to gradually align common features and effectively enhance the complementary features between modalities. By analyzing the generated connectivity features, the proposed model can identify AD-related brain connections. Evaluations on the public ADNI dataset show that the proposed CT-GAN can dramatically improve prediction performance and detect AD-related brain regions effectively. The proposed model also provides new insights for detecting AD-related abnormal neural circuits.
Feature-selected Graph Spatial Attention Network for Addictive Brain-Networks Identification
Jul 05, 2022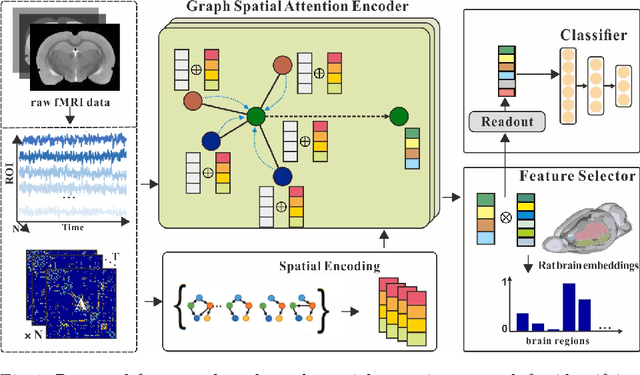

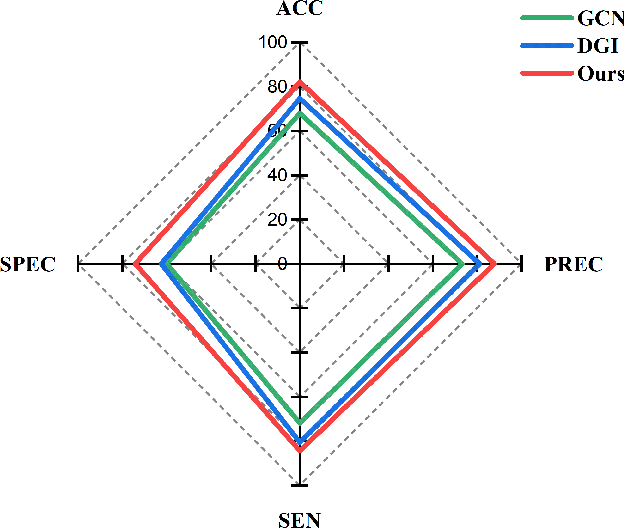
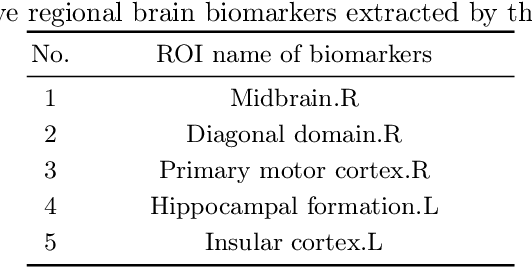
Abstract:Functional alterations in the relevant neural circuits occur from drug addiction over a certain period. And these significant alterations are also revealed by analyzing fMRI. However, because of fMRI's high dimensionality and poor signal-to-noise ratio, it is challenging to encode efficient and robust brain regional embeddings for both graph-level identification and region-level biomarkers detection tasks between nicotine addiction (NA) and healthy control (HC) groups. In this work, we represent the fMRI of the rat brain as a graph with biological attributes and propose a novel feature-selected graph spatial attention network(FGSAN) to extract the biomarkers of addiction and identify from these brain networks. Specially, a graph spatial attention encoder is employed to capture the features of spatiotemporal brain networks with spatial information. The method simultaneously adopts a Bayesian feature selection strategy to optimize the model and improve classification task by constraining features. Experiments on an addiction-related neural imaging dataset show that the proposed model can obtain superior performance and detect interpretable biomarkers associated with addiction-relevant neural circuits.
Cross-Modal Transformer GAN: A Brain Structure-Function Deep Fusing Framework for Alzheimer's Disease
Jun 20, 2022



Abstract:Cross-modal fusion of different types of neuroimaging data has shown great promise for predicting the progression of Alzheimer's Disease(AD). However, most existing methods applied in neuroimaging can not efficiently fuse the functional and structural information from multi-modal neuroimages. In this work, a novel cross-modal transformer generative adversarial network(CT-GAN) is proposed to fuse functional information contained in resting-state functional magnetic resonance imaging (rs-fMRI) and structural information contained in Diffusion Tensor Imaging (DTI). The developed bi-attention mechanism can match functional information to structural information efficiently and maximize the capability of extracting complementary information from rs-fMRI and DTI. By capturing the deep complementary information between structural features and functional features, the proposed CT-GAN can detect the AD-related brain connectivity, which could be used as a bio-marker of AD. Experimental results show that the proposed model can not only improve classification performance but also detect the AD-related brain connectivity effectively.
DecGAN: Decoupling Generative Adversarial Network detecting abnormal neural circuits for Alzheimer's disease
Oct 12, 2021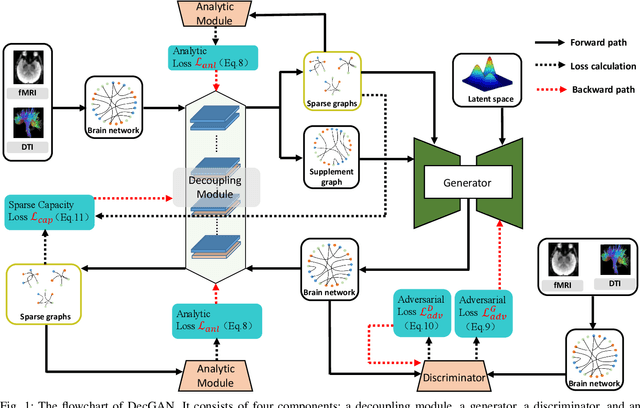
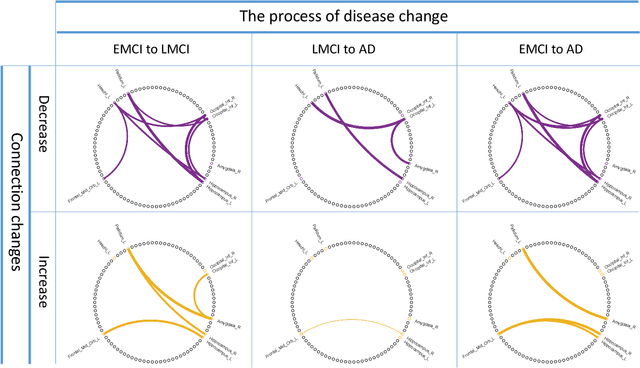
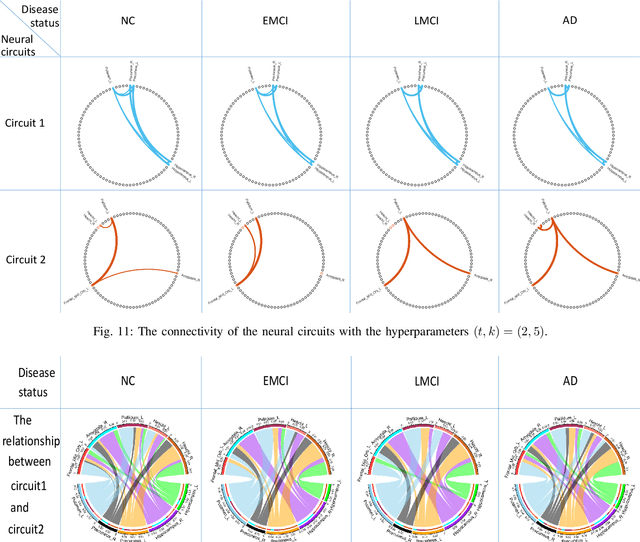
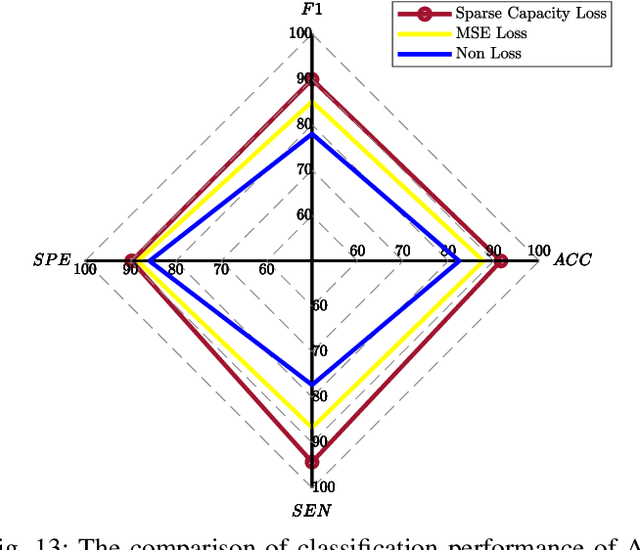
Abstract:One of the main reasons for Alzheimer's disease (AD) is the disorder of some neural circuits. Existing methods for AD prediction have achieved great success, however, detecting abnormal neural circuits from the perspective of brain networks is still a big challenge. In this work, a novel decoupling generative adversarial network (DecGAN) is proposed to detect abnormal neural circuits for AD. Concretely, a decoupling module is designed to decompose a brain network into two parts: one part is composed of a few sparse graphs which represent the neural circuits largely determining the development of AD; the other part is a supplement graph, whose influence on AD can be ignored. Furthermore, the adversarial strategy is utilized to guide the decoupling module to extract the feature more related to AD. Meanwhile, by encoding the detected neural circuits to hypergraph data, an analytic module associated with the hyperedge neurons algorithm is designed to identify the neural circuits. More importantly, a novel sparse capacity loss based on the spatial-spectral hypergraph similarity is developed to minimize the intrinsic topological distribution of neural circuits, which can significantly improve the accuracy and robustness of the proposed model. Experimental results demonstrate that the proposed model can effectively detect the abnormal neural circuits at different stages of AD, which is helpful for pathological study and early treatment.
Characterization Multimodal Connectivity of Brain Network by Hypergraph GAN for Alzheimer's Disease Analysis
Jul 21, 2021



Abstract:Using multimodal neuroimaging data to characterize brain network is currently an advanced technique for Alzheimer's disease(AD) Analysis. Over recent years the neuroimaging community has made tremendous progress in the study of resting-state functional magnetic resonance imaging (rs-fMRI) derived from blood-oxygen-level-dependent (BOLD) signals and Diffusion Tensor Imaging (DTI) derived from white matter fiber tractography. However, Due to the heterogeneity and complexity between BOLD signals and fiber tractography, Most existing multimodal data fusion algorithms can not sufficiently take advantage of the complementary information between rs-fMRI and DTI. To overcome this problem, a novel Hypergraph Generative Adversarial Networks(HGGAN) is proposed in this paper, which utilizes Interactive Hyperedge Neurons module (IHEN) and Optimal Hypergraph Homomorphism algorithm(OHGH) to generate multimodal connectivity of Brain Network from rs-fMRI combination with DTI. To evaluate the performance of this model, We use publicly available data from the ADNI database to demonstrate that the proposed model not only can identify discriminative brain regions of AD but also can effectively improve classification performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge