Benjamin Irving
Deep Semi-Supervised Embedded Clustering (DSEC) for Stratification of Heart Failure Patients
Jan 17, 2021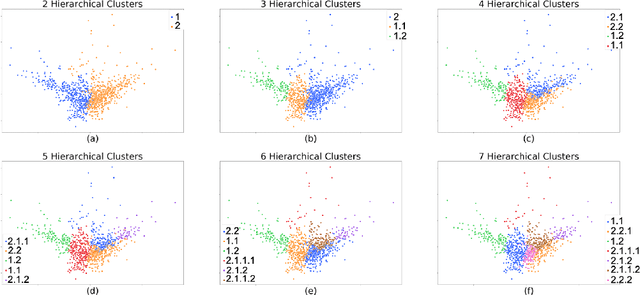
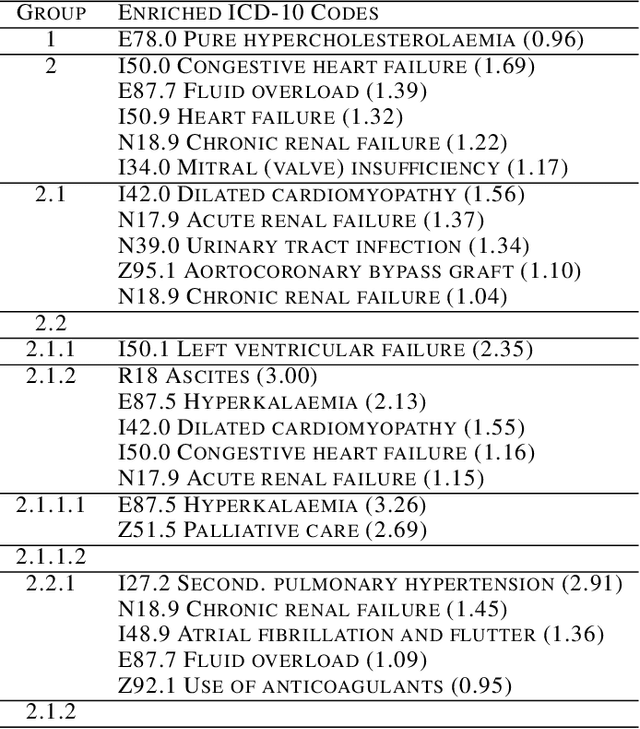
Abstract:Determining phenotypes of diseases can have considerable benefits for in-hospital patient care and to drug development. The structure of high dimensional data sets such as electronic health records are often represented through an embedding of the data, with clustering methods used to group data of similar structure. If subgroups are known to exist within data, supervised methods may be used to influence the clusters discovered. We propose to extend deep embedded clustering to a semi-supervised deep embedded clustering algorithm to stratify subgroups through known labels in the data. In this work we apply deep semi-supervised embedded clustering to determine data-driven patient subgroups of heart failure from the electronic health records of 4,487 heart failure and control patients. We find clinically relevant clusters from an embedded space derived from heterogeneous data. The proposed algorithm can potentially find new undiagnosed subgroups of patients that have different outcomes, and, therefore, lead to improved treatments.
Prediction of the onset of cardiovascular diseases from electronic health records using multi-task gated recurrent units
Jul 16, 2020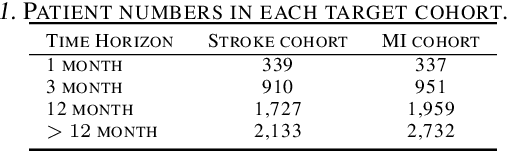
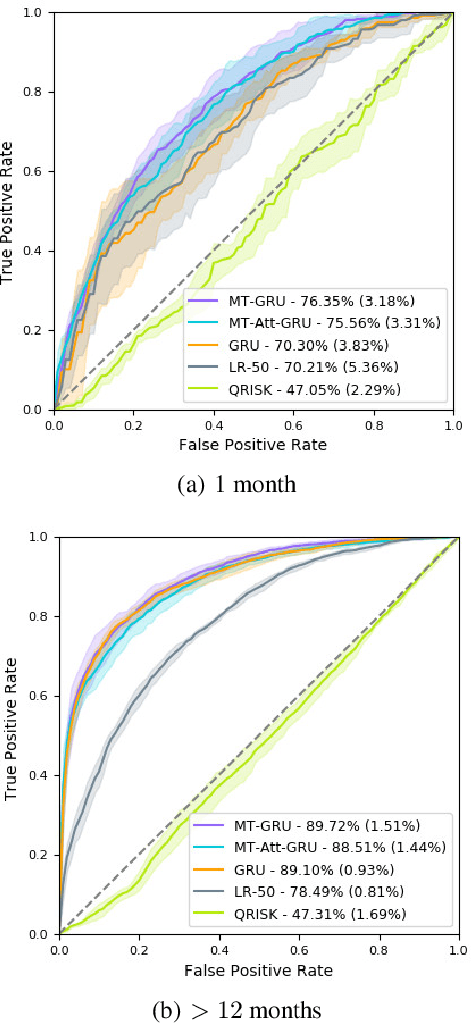
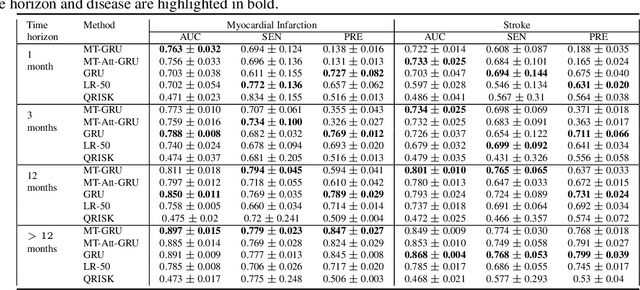
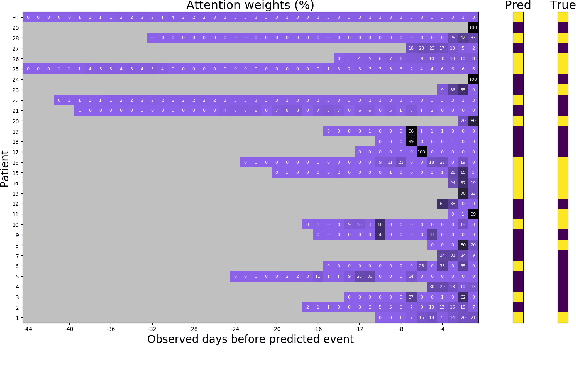
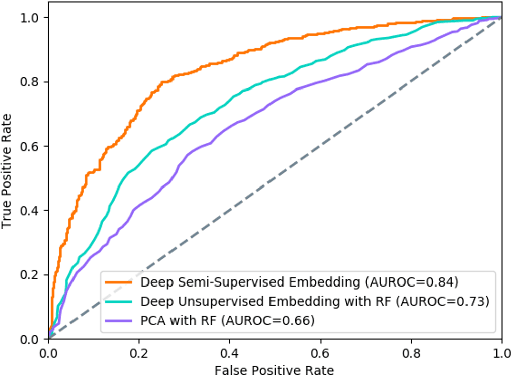
Abstract:In this work, we propose a multi-task recurrent neural network with attention mechanism for predicting cardiovascular events from electronic health records (EHRs) at different time horizons. The proposed approach is compared to a standard clinical risk predictor (QRISK) and machine learning alternatives using 5-year data from a NHS Foundation Trust. The proposed model outperforms standard clinical risk scores in predicting stroke (AUC=0.85) and myocardial infarction (AUC=0.89), considering the largest time horizon. Benefit of using an \gls{mt} setting becomes visible for very short time horizons, which results in an AUC increase between 2-6%. Further, we explored the importance of individual features and attention weights in predicting cardiovascular events. Our results indicate that the recurrent neural network approach benefits from the hospital longitudinal information and demonstrates how machine learning techniques can be applied to secondary care.
Extracting 3D Vascular Structures from Microscopy Images using Convolutional Recurrent Networks
May 26, 2017



Abstract:Vasculature is known to be of key biological significance, especially in the study of cancer. As such, considerable effort has been focused on the automated measurement and analysis of vasculature in medical and pre-clinical images. In tumors in particular, the vascular networks may be extremely irregular and the appearance of the individual vessels may not conform to classical descriptions of vascular appearance. Typically, vessels are extracted by either a segmentation and thinning pipeline, or by direct tracking. Neither of these methods are well suited to microscopy images of tumor vasculature. In order to address this we propose a method to directly extract a medial representation of the vessels using Convolutional Neural Networks. We then show that these two-dimensional centerlines can be meaningfully extended into 3D in anisotropic and complex microscopy images using the recently popularized Convolutional Long Short-Term Memory units (ConvLSTM). We demonstrate the effectiveness of this hybrid convolutional-recurrent architecture over both 2D and 3D convolutional comparators.
maskSLIC: Regional Superpixel Generation with Application to Local Pathology Characterisation in Medical Images
Feb 09, 2017



Abstract:Supervoxel methods such as Simple Linear Iterative Clustering (SLIC) are an effective technique for partitioning an image or volume into locally similar regions, and are a common building block for the development of detection, segmentation and analysis methods. We introduce maskSLIC an extension of SLIC to create supervoxels within regions-of-interest, and demonstrate,on examples from 2-dimensions to 4-dimensions, that maskSLIC overcomes issues that affect SLIC within an irregular mask. We highlight the benefits of this method through examples, and show that it is able to better represent underlying tumour subregions and achieves significantly better results than SLIC on the BRATS 2013 brain tumour challenge data (p=0.001) - outperforming SLIC on 18/20 scans. Finally, we show an application of this method for the analysis of functional tumour subregions and demonstrate that it is more effective than voxel clustering.
Pieces-of-parts for supervoxel segmentation with global context: Application to DCE-MRI tumour delineation
Apr 18, 2016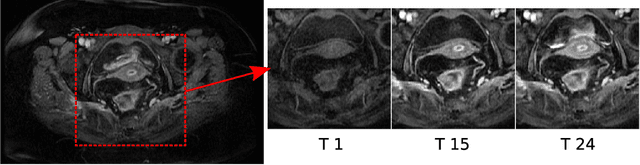

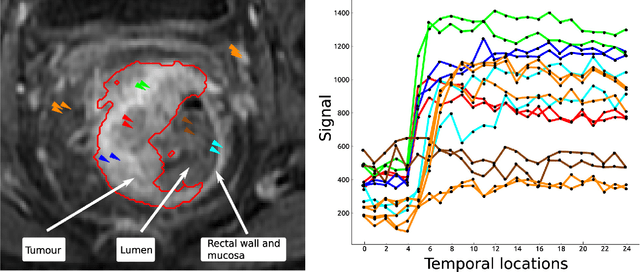
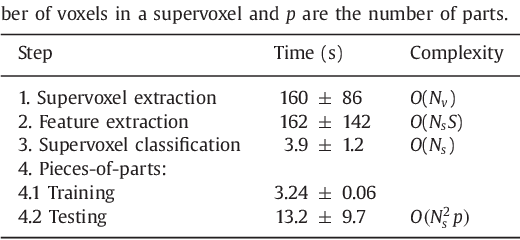
Abstract:Rectal tumour segmentation in dynamic contrast-enhanced MRI (DCE-MRI) is a challenging task, and an automated and consistent method would be highly desirable to improve the modelling and prediction of patient outcomes from tissue contrast enhancement characteristics - particularly in routine clinical practice. A framework is developed to automate DCE-MRI tumour segmentation, by introducing: perfusion-supervoxels to over-segment and classify DCE-MRI volumes using the dynamic contrast enhancement characteristics; and the pieces-of-parts graphical model, which adds global (anatomic) constraints that further refine the supervoxel components that comprise the tumour. The framework was evaluated on 23 DCE-MRI scans of patients with rectal adenocarcinomas, and achieved a voxelwise area-under the receiver operating characteristic curve (AUC) of 0.97 compared to expert delineations. Creating a binary tumour segmentation, 21 of the 23 cases were segmented correctly with a median Dice similarity coefficient (DSC) of 0.63, which is close to the inter-rater variability of this challenging task. A sec- ond study is also included to demonstrate the method's generalisability and achieved a DSC of 0.71. The framework achieves promising results for the underexplored area of rectal tumour segmentation in DCE-MRI, and the methods have potential to be applied to other DCE-MRI and supervoxel segmentation problems
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge