Julia A Schnabel
Reversing the Abnormal: Pseudo-Healthy Generative Networks for Anomaly Detection
Mar 15, 2023Abstract:Early and accurate disease detection is crucial for patient management and successful treatment outcomes. However, the automatic identification of anomalies in medical images can be challenging. Conventional methods rely on large labeled datasets which are difficult to obtain. To overcome these limitations, we introduce a novel unsupervised approach, called PHANES (Pseudo Healthy generative networks for ANomaly Segmentation). Our method has the capability of reversing anomalies, i.e., preserving healthy tissue and replacing anomalous regions with pseudo-healthy (PH) reconstructions. Unlike recent diffusion models, our method does not rely on a learned noise distribution nor does it introduce random alterations to the entire image. Instead, we use latent generative networks to create masks around possible anomalies, which are refined using inpainting generative networks. We demonstrate the effectiveness of PHANES in detecting stroke lesions in T1w brain MRI datasets and show significant improvements over state-of-the-art (SOTA) methods. We believe that our proposed framework will open new avenues for interpretable, fast, and accurate anomaly segmentation with the potential to support various clinical-oriented downstream tasks.
Pieces-of-parts for supervoxel segmentation with global context: Application to DCE-MRI tumour delineation
Apr 18, 2016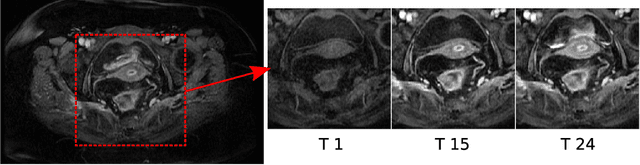

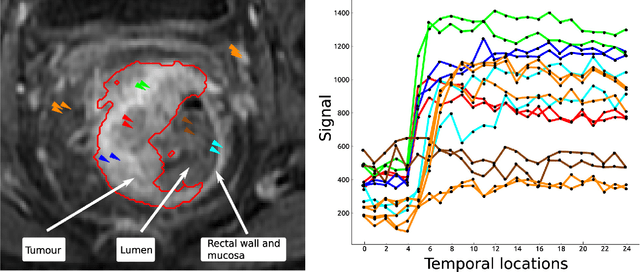
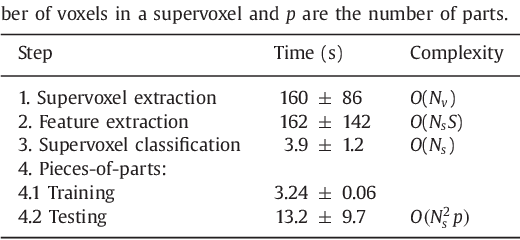
Abstract:Rectal tumour segmentation in dynamic contrast-enhanced MRI (DCE-MRI) is a challenging task, and an automated and consistent method would be highly desirable to improve the modelling and prediction of patient outcomes from tissue contrast enhancement characteristics - particularly in routine clinical practice. A framework is developed to automate DCE-MRI tumour segmentation, by introducing: perfusion-supervoxels to over-segment and classify DCE-MRI volumes using the dynamic contrast enhancement characteristics; and the pieces-of-parts graphical model, which adds global (anatomic) constraints that further refine the supervoxel components that comprise the tumour. The framework was evaluated on 23 DCE-MRI scans of patients with rectal adenocarcinomas, and achieved a voxelwise area-under the receiver operating characteristic curve (AUC) of 0.97 compared to expert delineations. Creating a binary tumour segmentation, 21 of the 23 cases were segmented correctly with a median Dice similarity coefficient (DSC) of 0.63, which is close to the inter-rater variability of this challenging task. A sec- ond study is also included to demonstrate the method's generalisability and achieved a DSC of 0.71. The framework achieves promising results for the underexplored area of rectal tumour segmentation in DCE-MRI, and the methods have potential to be applied to other DCE-MRI and supervoxel segmentation problems
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge