Andre van der Kouwe
Learning Task-Specific Strategies for Accelerated MRI
Apr 25, 2023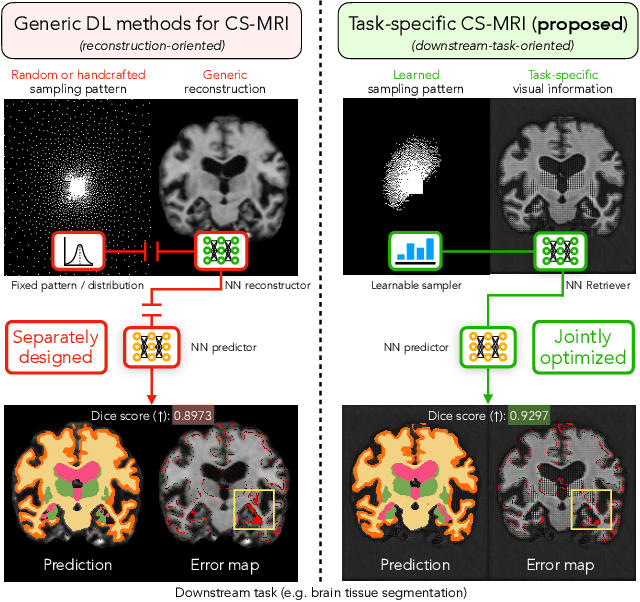
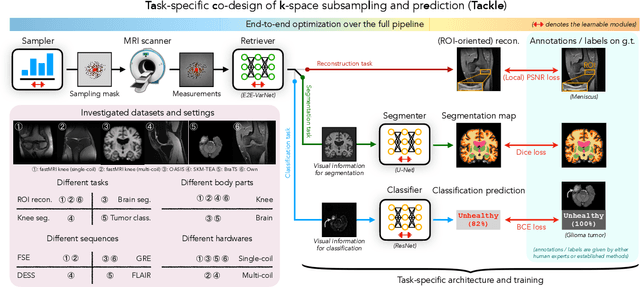

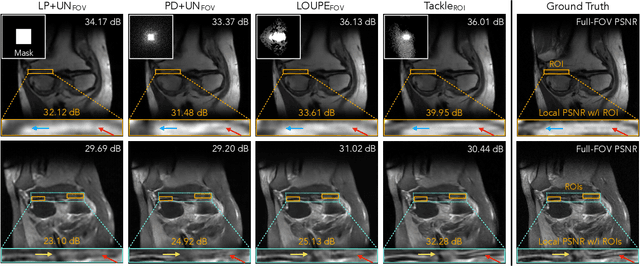
Abstract:Compressed sensing magnetic resonance imaging (CS-MRI) seeks to recover visual information from subsampled measurements for diagnostic tasks. Traditional CS-MRI methods often separately address measurement subsampling, image reconstruction, and task prediction, resulting in suboptimal end-to-end performance. In this work, we propose TACKLE as a unified framework for designing CS-MRI systems tailored to specific tasks. Leveraging recent co-design techniques, TACKLE jointly optimizes subsampling, reconstruction, and prediction strategies to enhance the performance on the downstream task. Our results on multiple public MRI datasets show that the proposed framework achieves improved performance on various tasks over traditional CS-MRI methods. We also evaluate the generalization ability of TACKLE by experimentally collecting a new dataset using different acquisition setups from the training data. Without additional fine-tuning, TACKLE functions robustly and leads to both numerical and visual improvements.
Unsupervised learning of MRI tissue properties using MRI physics models
Jul 06, 2021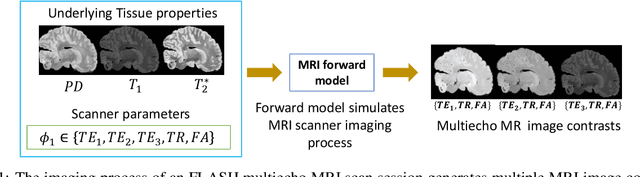

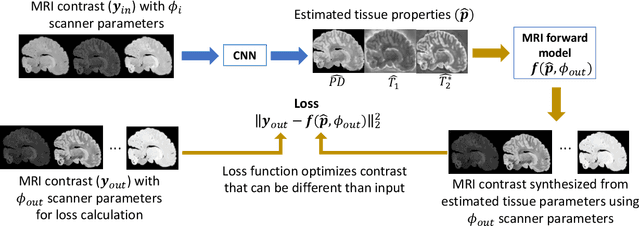
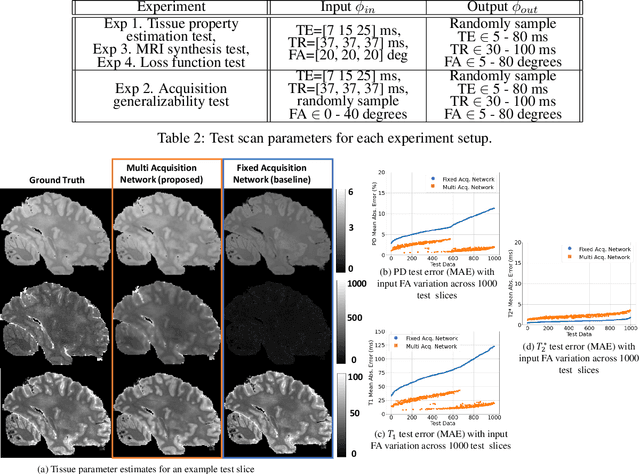
Abstract:In neuroimaging, MRI tissue properties characterize underlying neurobiology, provide quantitative biomarkers for neurological disease detection and analysis, and can be used to synthesize arbitrary MRI contrasts. Estimating tissue properties from a single scan session using a protocol available on all clinical scanners promises to reduce scan time and cost, enable quantitative analysis in routine clinical scans and provide scan-independent biomarkers of disease. However, existing tissue properties estimation methods - most often $\mathbf{T_1}$ relaxation, $\mathbf{T_2^*}$ relaxation, and proton density ($\mathbf{PD}$) - require data from multiple scan sessions and cannot estimate all properties from a single clinically available MRI protocol such as the multiecho MRI scan. In addition, the widespread use of non-standard acquisition parameters across clinical imaging sites require estimation methods that can generalize across varying scanner parameters. However, existing learning methods are acquisition protocol specific and cannot estimate from heterogenous clinical data from different imaging sites. In this work we propose an unsupervised deep-learning strategy that employs MRI physics to estimate all three tissue properties from a single multiecho MRI scan session, and generalizes across varying acquisition parameters. The proposed strategy optimizes accurate synthesis of new MRI contrasts from estimated latent tissue properties, enabling unsupervised training, we also employ random acquisition parameters during training to achieve acquisition generalization. We provide the first demonstration of estimating all tissue properties from a single multiecho scan session. We demonstrate improved accuracy and generalizability for tissue property estimation and MRI synthesis.
Fast Infant MRI Skullstripping with Multiview 2D Convolutional Neural Networks
Apr 27, 2019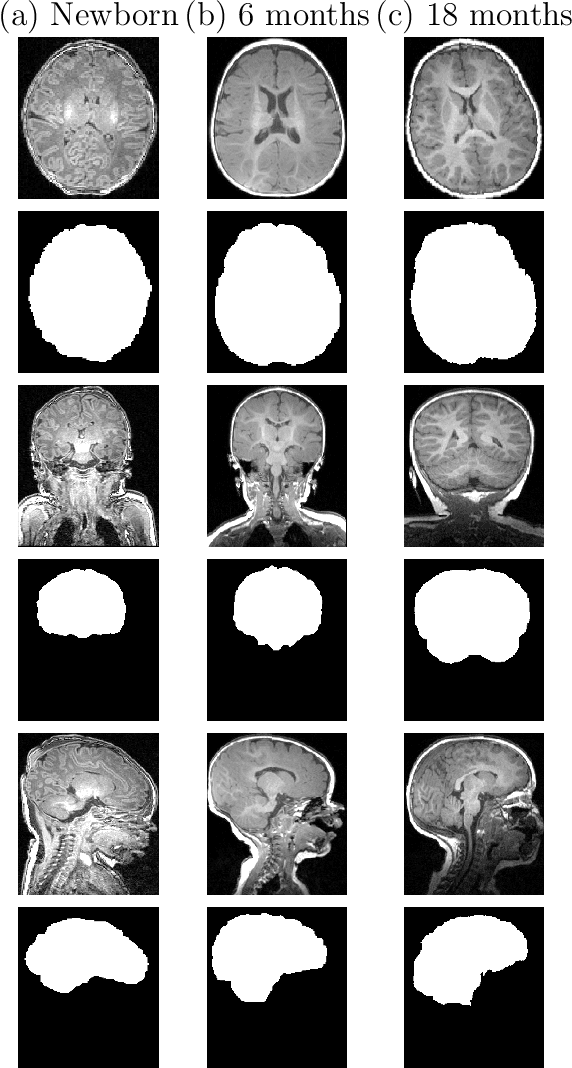
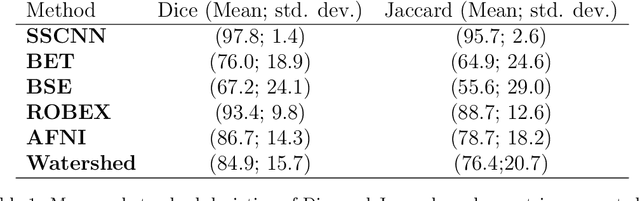
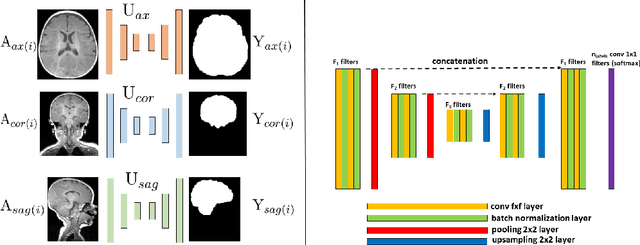
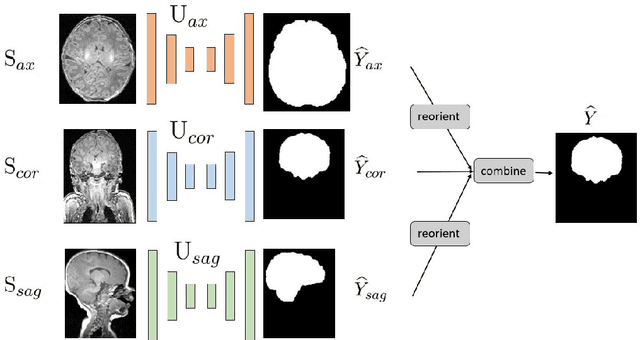
Abstract:Skullstripping is defined as the task of segmenting brain tissue from a full head magnetic resonance image~(MRI). It is a critical component in neuroimage processing pipelines. Downstream deformable registration and whole brain segmentation performance is highly dependent on accurate skullstripping. Skullstripping is an especially challenging task for infant~(age range 0--18 months) head MRI images due to the significant size and shape variability of the head and the brain in that age range. Infant brain tissue development also changes the $T_1$-weighted image contrast over time, making consistent skullstripping a difficult task. Existing tools for adult brain MRI skullstripping are ill equipped to handle these variations and a specialized infant MRI skullstripping algorithm is necessary. In this paper, we describe a supervised skullstripping algorithm that utilizes three trained fully convolutional neural networks~(CNN), each of which segments 2D $T_1$-weighted slices in axial, coronal, and sagittal views respectively. The three probabilistic segmentations in the three views are linearly fused and thresholded to produce a final brain mask. We compared our method to existing adult and infant skullstripping algorithms and showed significant improvement based on Dice overlap metric~(average Dice of 0.97) with a manually labeled ground truth data set. Label fusion experiments on multiple, unlabeled data sets show that our method is consistent and has fewer failure modes. In addition, our method is computationally very fast with a run time of 30 seconds per image on NVidia P40/P100/Quadro 4000 GPUs.
A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology
Jun 22, 2018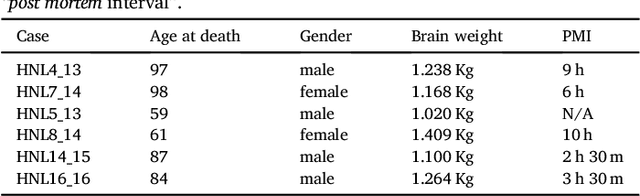
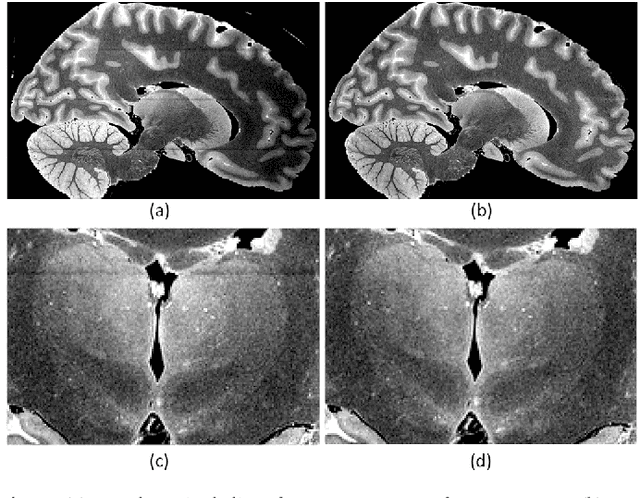
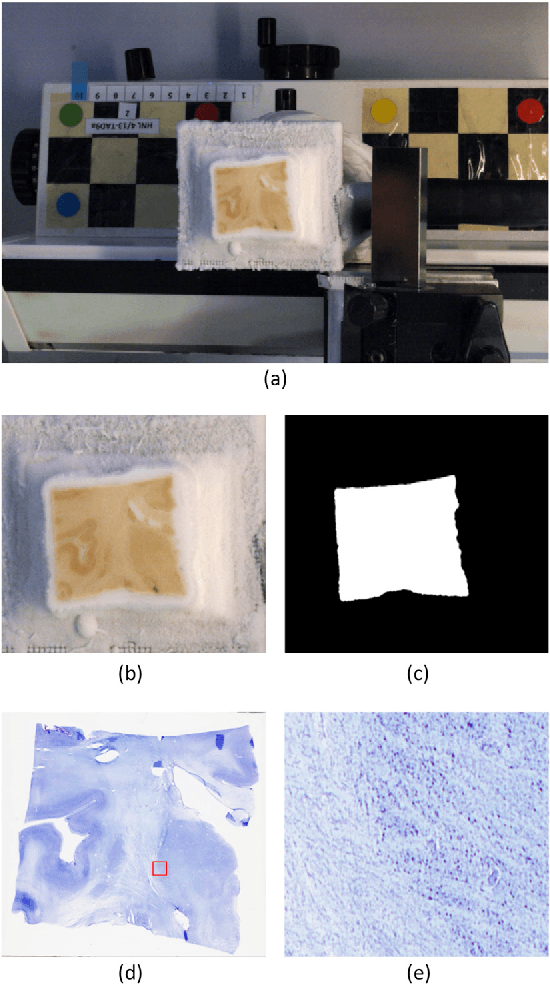
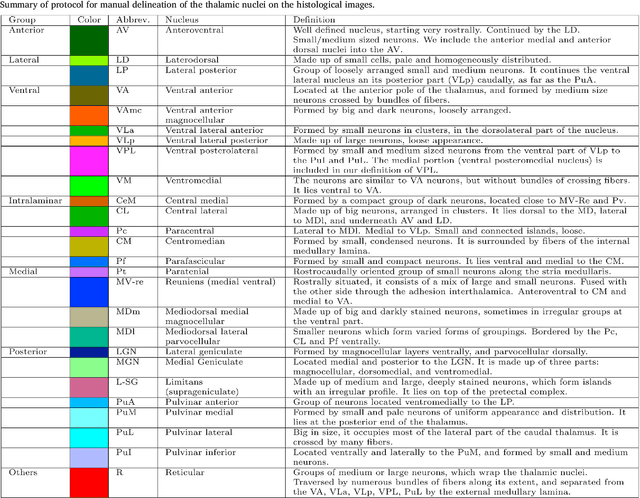
Abstract:The human thalamus is a brain structure that comprises numerous, highly specific nuclei. Since these nuclei are known to have different functions and to be connected to different areas of the cerebral cortex, it is of great interest for the neuroimaging community to study their volume, shape and connectivity in vivo with MRI. In this study, we present a probabilistic atlas of the thalamic nuclei built using ex vivo brain MRI scans and histological data, as well as the application of the atlas to in vivo MRI segmentation. The atlas was built using manual delineation of 26 thalamic nuclei on the serial histology of 12 whole thalami from six autopsy samples, combined with manual segmentations of the whole thalamus and surrounding structures (caudate, putamen, hippocampus, etc.) made on in vivo brain MR data from 39 subjects. The 3D structure of the histological data and corresponding manual segmentations was recovered using the ex vivo MRI as reference frame, and stacks of blockface photographs acquired during the sectioning as intermediate target. The atlas, which was encoded as an adaptive tetrahedral mesh, shows a good agreement with with previous histological studies of the thalamus in terms of volumes of representative nuclei. When applied to segmentation of in vivo scans using Bayesian inference, the atlas shows excellent test-retest reliability, robustness to changes in input MRI contrast, and ability to detect differential thalamic effects in subjects with Alzheimer's disease. The probabilistic atlas and companion segmentation tool are publicly available as part of the neuroimaging package FreeSurfer.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge