Ali Ertürk
Panoptica -- instance-wise evaluation of 3D semantic and instance segmentation maps
Dec 05, 2023



Abstract:This paper introduces panoptica, a versatile and performance-optimized package designed for computing instance-wise segmentation quality metrics from 2D and 3D segmentation maps. panoptica addresses the limitations of existing metrics and provides a modular framework that complements the original intersection over union-based panoptic quality with other metrics, such as the distance metric Average Symmetric Surface Distance. The package is open-source, implemented in Python, and accompanied by comprehensive documentation and tutorials. panoptica employs a three-step metrics computation process to cover diverse use cases. The efficacy of panoptica is demonstrated on various real-world biomedical datasets, where an instance-wise evaluation is instrumental for an accurate representation of the underlying clinical task. Overall, we envision panoptica as a valuable tool facilitating in-depth evaluation of segmentation methods.
Approaching Peak Ground Truth
Dec 31, 2022

Abstract:Machine learning models are typically evaluated by computing similarity with reference annotations and trained by maximizing similarity with such. Especially in the bio-medical domain, annotations are subjective and suffer from low inter- and intra-rater reliability. Since annotations only reflect the annotation entity's interpretation of the real world, this can lead to sub-optimal predictions even though the model achieves high similarity scores. Here, the theoretical concept of Peak Ground Truth (PGT) is introduced. PGT marks the point beyond which an increase in similarity with the reference annotation stops translating to better Real World Model Performance (RWMP). Additionally, a quantitative technique to approximate PGT by computing inter- and intra-rater reliability is proposed. Finally, three categories of PGT-aware strategies to evaluate and improve model performance are reviewed.
Whole Brain Vessel Graphs: A Dataset and Benchmark for Graph Learning and Neuroscience
Aug 30, 2021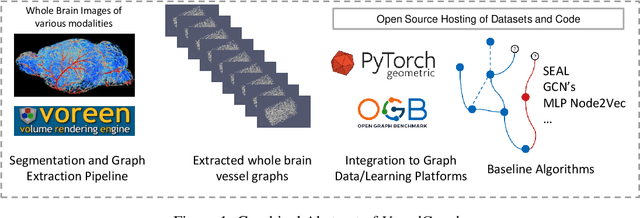
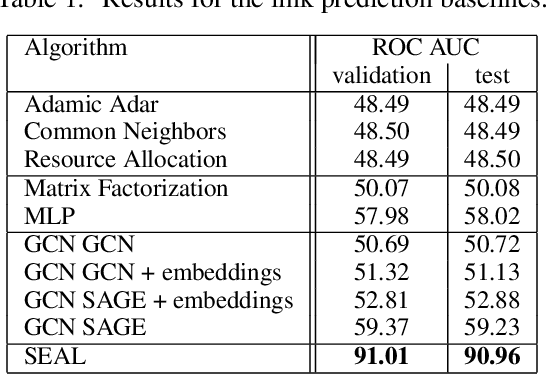
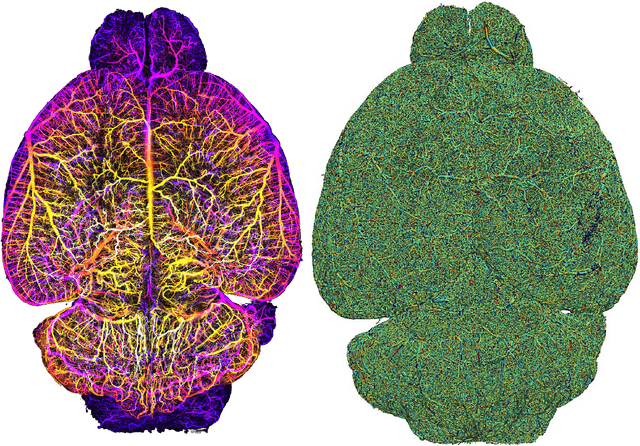
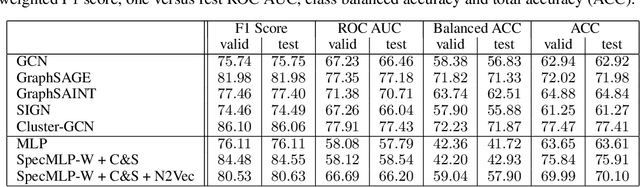
Abstract:Biological neural networks define the brain function and intelligence of humans and other mammals, and form ultra-large, spatial, structured graphs. Their neuronal organization is closely interconnected with the spatial organization of the brain's microvasculature, which supplies oxygen to the neurons and builds a complementary spatial graph. This vasculature (or the vessel structure) plays an important role in neuroscience; for example, the organization of (and changes to) vessel structure can represent early signs of various pathologies, e.g. Alzheimer's disease or stroke. Recently, advances in tissue clearing have enabled whole brain imaging and segmentation of the entirety of the mouse brain's vasculature. Building on these advances in imaging, we are presenting an extendable dataset of whole-brain vessel graphs based on specific imaging protocols. Specifically, we extract vascular graphs using a refined graph extraction scheme leveraging the volume rendering engine Voreen and provide them in an accessible and adaptable form through the OGB and PyTorch Geometric dataloaders. Moreover, we benchmark numerous state-of-the-art graph learning algorithms on the biologically relevant tasks of vessel prediction and vessel classification using the introduced vessel graph dataset. Our work paves a path towards advancing graph learning research into the field of neuroscience. Complementarily, the presented dataset raises challenging graph learning research questions for the machine learning community, in terms of incorporating biological priors into learning algorithms, or in scaling these algorithms to handle sparse,spatial graphs with millions of nodes and edges. All datasets and code are available for download at https://github.com/jocpae/VesselGraph .
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge