Alan Morris
HAMIL-QA: Hierarchical Approach to Multiple Instance Learning for Atrial LGE MRI Quality Assessment
Jul 09, 2024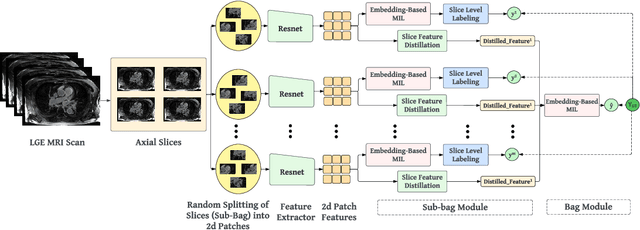
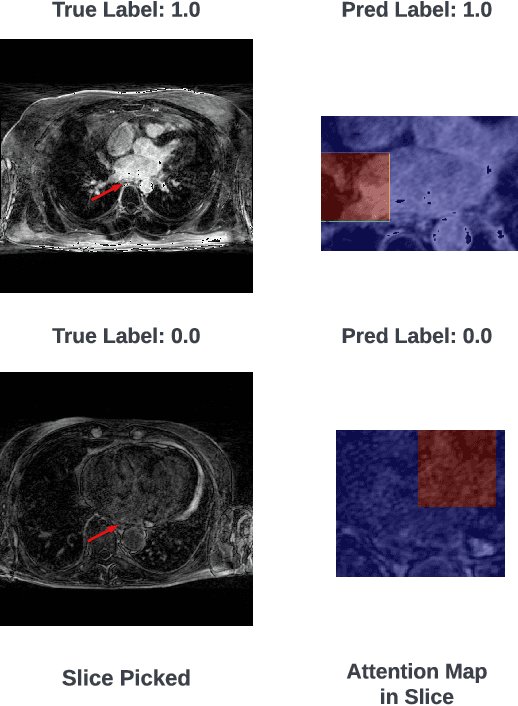
Abstract:The accurate evaluation of left atrial fibrosis via high-quality 3D Late Gadolinium Enhancement (LGE) MRI is crucial for atrial fibrillation management but is hindered by factors like patient movement and imaging variability. The pursuit of automated LGE MRI quality assessment is critical for enhancing diagnostic accuracy, standardizing evaluations, and improving patient outcomes. The deep learning models aimed at automating this process face significant challenges due to the scarcity of expert annotations, high computational costs, and the need to capture subtle diagnostic details in highly variable images. This study introduces HAMIL-QA, a multiple instance learning (MIL) framework, designed to overcome these obstacles. HAMIL-QA employs a hierarchical bag and sub-bag structure that allows for targeted analysis within sub-bags and aggregates insights at the volume level. This hierarchical MIL approach reduces reliance on extensive annotations, lessens computational load, and ensures clinically relevant quality predictions by focusing on diagnostically critical image features. Our experiments show that HAMIL-QA surpasses existing MIL methods and traditional supervised approaches in accuracy, AUROC, and F1-Score on an LGE MRI scan dataset, demonstrating its potential as a scalable solution for LGE MRI quality assessment automation. The code is available at: $\href{https://github.com/arf111/HAMIL-QA}{\text{this https URL}}$
Particle-Based Shape Modeling for Arbitrary Regions-of-Interest
Dec 29, 2023Abstract:Statistical Shape Modeling (SSM) is a quantitative method for analyzing morphological variations in anatomical structures. These analyses often necessitate building models on targeted anatomical regions of interest to focus on specific morphological features. We propose an extension to \particle-based shape modeling (PSM), a widely used SSM framework, to allow shape modeling to arbitrary regions of interest. Existing methods to define regions of interest are computationally expensive and have topological limitations. To address these shortcomings, we use mesh fields to define free-form constraints, which allow for delimiting arbitrary regions of interest on shape surfaces. Furthermore, we add a quadratic penalty method to the model optimization to enable computationally efficient enforcement of any combination of cutting-plane and free-form constraints. We demonstrate the effectiveness of this method on a challenging synthetic dataset and two medical datasets.
Two-Stage Deep Learning Framework for Quality Assessment of Left Atrial Late Gadolinium Enhanced MRI Images
Oct 13, 2023Abstract:Accurate assessment of left atrial fibrosis in patients with atrial fibrillation relies on high-quality 3D late gadolinium enhancement (LGE) MRI images. However, obtaining such images is challenging due to patient motion, changing breathing patterns, or sub-optimal choice of pulse sequence parameters. Automated assessment of LGE-MRI image diagnostic quality is clinically significant as it would enhance diagnostic accuracy, improve efficiency, ensure standardization, and contributes to better patient outcomes by providing reliable and high-quality LGE-MRI scans for fibrosis quantification and treatment planning. To address this, we propose a two-stage deep-learning approach for automated LGE-MRI image diagnostic quality assessment. The method includes a left atrium detector to focus on relevant regions and a deep network to evaluate diagnostic quality. We explore two training strategies, multi-task learning, and pretraining using contrastive learning, to overcome limited annotated data in medical imaging. Contrastive Learning result shows about $4\%$, and $9\%$ improvement in F1-Score and Specificity compared to Multi-Task learning when there's limited data.
Statistical Shape Modeling of Biventricular Anatomy with Shared Boundaries
Sep 12, 2022



Abstract:Statistical shape modeling (SSM) is a valuable and powerful tool to generate a detailed representation of complex anatomy that enables quantitative analysis and the comparison of shapes and their variations. SSM applies mathematics, statistics, and computing to parse the shape into a quantitative representation (such as correspondence points or landmarks) that will help answer various questions about the anatomical variations across the population. Complex anatomical structures have many diverse parts with varying interactions or intricate architecture. For example, the heart is four-chambered anatomy with several shared boundaries between chambers. Coordinated and efficient contraction of the chambers of the heart is necessary to adequately perfuse end organs throughout the body. Subtle shape changes within these shared boundaries of the heart can indicate potential pathological changes that lead to uncoordinated contraction and poor end-organ perfusion. Early detection and robust quantification could provide insight into ideal treatment techniques and intervention timing. However, existing SSM approaches fall short of explicitly modeling the statistics of shared boundaries. This paper presents a general and flexible data-driven approach for building statistical shape models of multi-organ anatomies with shared boundaries that capture morphological and alignment changes of individual anatomies and their shared boundary surfaces throughout the population. We demonstrate the effectiveness of the proposed methods using a biventricular heart dataset by developing shape models that consistently parameterize the cardiac biventricular structure and the interventricular septum (shared boundary surface) across the population data.
Spatiotemporal Cardiac Statistical Shape Modeling: A Data-Driven Approach
Sep 06, 2022



Abstract:Clinical investigations of anatomy's structural changes over time could greatly benefit from population-level quantification of shape, or spatiotemporal statistic shape modeling (SSM). Such a tool enables characterizing patient organ cycles or disease progression in relation to a cohort of interest. Constructing shape models requires establishing a quantitative shape representation (e.g., corresponding landmarks). Particle-based shape modeling (PSM) is a data-driven SSM approach that captures population-level shape variations by optimizing landmark placement. However, it assumes cross-sectional study designs and hence has limited statistical power in representing shape changes over time. Existing methods for modeling spatiotemporal or longitudinal shape changes require predefined shape atlases and pre-built shape models that are typically constructed cross-sectionally. This paper proposes a data-driven approach inspired by the PSM method to learn population-level spatiotemporal shape changes directly from shape data. We introduce a novel SSM optimization scheme that produces landmarks that are in correspondence both across the population (inter-subject) and across time-series (intra-subject). We apply the proposed method to 4D cardiac data from atrial-fibrillation patients and demonstrate its efficacy in representing the dynamic change of the left atrium. Furthermore, we show that our method outperforms an image-based approach for spatiotemporal SSM with respect to a generative time-series model, the Linear Dynamical System (LDS). LDS fit using a spatiotemporal shape model optimized via our approach provides better generalization and specificity, indicating it accurately captures the underlying time-dependency.
Benchmarking off-the-shelf statistical shape modeling tools in clinical applications
Sep 07, 2020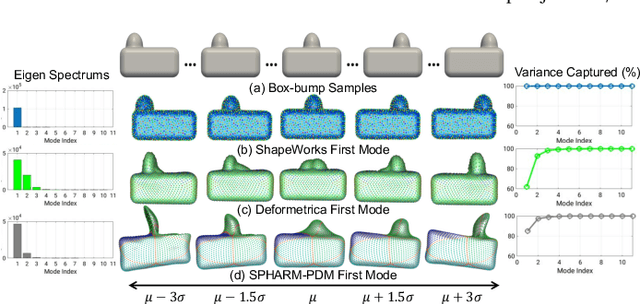

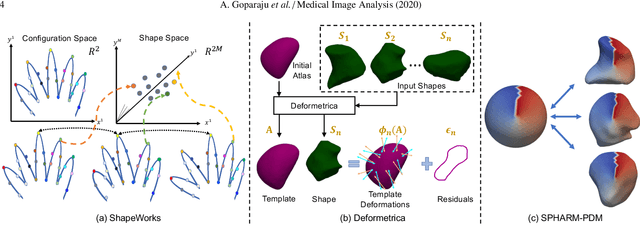
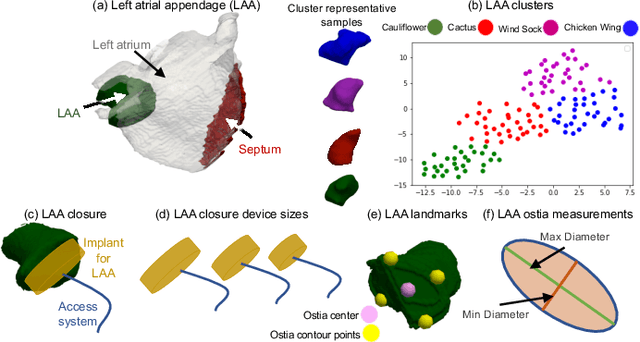
Abstract:Statistical shape modeling (SSM) is widely used in biology and medicine as a new generation of morphometric approaches for the quantitative analysis of anatomical shapes. Technological advancements of in vivo imaging have led to the development of open-source computational tools that automate the modeling of anatomical shapes and their population-level variability. However, little work has been done on the evaluation and validation of such tools in clinical applications that rely on morphometric quantifications (e.g., implant design and lesion screening). Here, we systematically assess the outcome of widely used, state-of-the-art SSM tools, namely ShapeWorks, Deformetrica, and SPHARM-PDM. We use both quantitative and qualitative metrics to evaluate shape models from different tools. We propose validation frameworks for anatomical landmark/measurement inference and lesion screening. We also present a lesion screening method to objectively characterize subtle abnormal shape changes with respect to learned population-level statistics of controls. Results demonstrate that SSM tools display different levels of consistencies, where ShapeWorks and Deformetrica models are more consistent compared to models from SPHARM-PDM due to the groupwise approach of estimating surface correspondences. Furthermore, ShapeWorks and Deformetrica shape models are found to capture clinically relevant population-level variability compared to SPHARM-PDM models.
On the Evaluation and Validation of Off-the-shelf Statistical Shape Modeling Tools: A Clinical Application
Oct 03, 2018



Abstract:Statistical shape modeling (SSM) has proven useful in many areas of biology and medicine as a new generation of morphometric approaches for the quantitative analysis of anatomical shapes. Recently, the increased availability of high-resolution in vivo images of anatomy has led to the development and distribution of open-source computational tools to model anatomical shapes and their variability within populations with unprecedented detail and statistical power. Nonetheless, there is little work on the evaluation and validation of such tools as related to clinical applications that rely on morphometric quantifications for treatment planning. To address this lack of validation, we systematically assess the outcome of widely used off-the-shelf SSM tools, namely ShapeWorks, SPHARM-PDM, and Deformetrica, in the context of designing closure devices for left atrium appendage (LAA) in atrial fibrillation (AF) patients to prevent stroke, where an incomplete LAA closure may be worse than no closure. This study is motivated by the potential role of SSM in the geometric design of closure devices, which could be informed by population-level statistics, and patient-specific device selection, which is driven by anatomical measurements that could be automated by relating patient-level anatomy to population-level morphometrics. Hence, understanding the consequences of different SSM tools for the final analysis is critical for the careful choice of the tool to be deployed in real clinical scenarios. Results demonstrate that estimated measurements from ShapeWorks model are more consistent compared to models from Deformetrica and SPHARM-PDM. Furthermore, ShapeWorks and Deformetrica shape models capture clinically relevant population-level variability compared to SPHARM-PDM models.
Deep Learning for End-to-End Atrial Fibrillation Recurrence Estimation
Sep 30, 2018



Abstract:Left atrium shape has been shown to be an independent predictor of recurrence after atrial fibrillation (AF) ablation. Shape-based representation is imperative to such an estimation process, where correspondence-based representation offers the most flexibility and ease-of-computation for population-level shape statistics. Nonetheless, population-level shape representations in the form of image segmentation and correspondence models derived from cardiac MRI require significant human resources with sufficient anatomy-specific expertise. In this paper, we propose a machine learning approach that uses deep networks to estimate AF recurrence by predicting shape descriptors directly from MRI images, with NO image pre-processing involved. We also propose a novel data augmentation scheme to effectively train a deep network in a limited training data setting. We compare this new method of estimating shape descriptors from images with the state-of-the-art correspondence-based shape modeling that requires image segmentation and correspondence optimization. Results show that the proposed method and the current state-of-the-art produce statistically similar outcomes on AF recurrence, eliminating the need for expensive pre-processing pipelines and associated human labor.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge