K M Arefeen Sultan
EF-Net: A Deep Learning Approach Combining Word Embeddings and Feature Fusion for Patient Disposition Analysis
Dec 20, 2024



Abstract:One of the most urgent problems is the overcrowding in emergency departments (EDs), caused by an aging population and rising healthcare costs. Patient dispositions have become more complex as a result of the strain on hospital infrastructure and the scarcity of medical resources. Individuals with more dangerous health issues should be prioritized in the emergency room. Thus, our research aims to develop a prediction model for patient disposition using EF-Net. This model will incorporate categorical features into the neural network layer and add numerical features with the embedded categorical features. We combine the EF-Net and XGBoost models to attain higher accuracy in our results. The result is generated using the soft voting technique. In EF-Net, we attained an accuracy of 95.33%, whereas in the Ensemble Model, we achieved an accuracy of 96%. The experiment's analysis shows that EF-Net surpasses existing works in accuracy, AUROC, and F1-Score on the MIMIC-IV-ED dataset, demonstrating its potential as a scalable solution for patient disposition assessment. Our code is available at https://github.com/nafisa67/thesis
HAMIL-QA: Hierarchical Approach to Multiple Instance Learning for Atrial LGE MRI Quality Assessment
Jul 09, 2024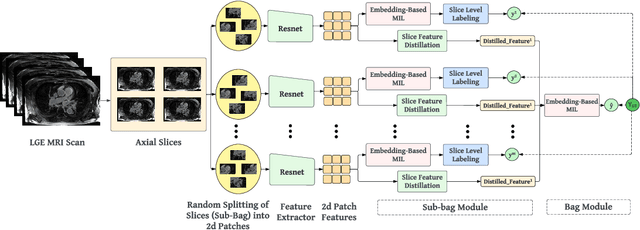
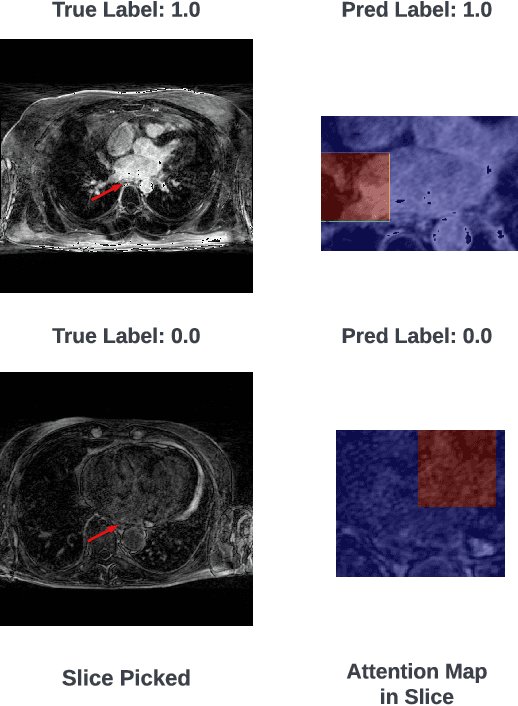
Abstract:The accurate evaluation of left atrial fibrosis via high-quality 3D Late Gadolinium Enhancement (LGE) MRI is crucial for atrial fibrillation management but is hindered by factors like patient movement and imaging variability. The pursuit of automated LGE MRI quality assessment is critical for enhancing diagnostic accuracy, standardizing evaluations, and improving patient outcomes. The deep learning models aimed at automating this process face significant challenges due to the scarcity of expert annotations, high computational costs, and the need to capture subtle diagnostic details in highly variable images. This study introduces HAMIL-QA, a multiple instance learning (MIL) framework, designed to overcome these obstacles. HAMIL-QA employs a hierarchical bag and sub-bag structure that allows for targeted analysis within sub-bags and aggregates insights at the volume level. This hierarchical MIL approach reduces reliance on extensive annotations, lessens computational load, and ensures clinically relevant quality predictions by focusing on diagnostically critical image features. Our experiments show that HAMIL-QA surpasses existing MIL methods and traditional supervised approaches in accuracy, AUROC, and F1-Score on an LGE MRI scan dataset, demonstrating its potential as a scalable solution for LGE MRI quality assessment automation. The code is available at: $\href{https://github.com/arf111/HAMIL-QA}{\text{this https URL}}$
Two-Stage Deep Learning Framework for Quality Assessment of Left Atrial Late Gadolinium Enhanced MRI Images
Oct 13, 2023Abstract:Accurate assessment of left atrial fibrosis in patients with atrial fibrillation relies on high-quality 3D late gadolinium enhancement (LGE) MRI images. However, obtaining such images is challenging due to patient motion, changing breathing patterns, or sub-optimal choice of pulse sequence parameters. Automated assessment of LGE-MRI image diagnostic quality is clinically significant as it would enhance diagnostic accuracy, improve efficiency, ensure standardization, and contributes to better patient outcomes by providing reliable and high-quality LGE-MRI scans for fibrosis quantification and treatment planning. To address this, we propose a two-stage deep-learning approach for automated LGE-MRI image diagnostic quality assessment. The method includes a left atrium detector to focus on relevant regions and a deep network to evaluate diagnostic quality. We explore two training strategies, multi-task learning, and pretraining using contrastive learning, to overcome limited annotated data in medical imaging. Contrastive Learning result shows about $4\%$, and $9\%$ improvement in F1-Score and Specificity compared to Multi-Task learning when there's limited data.
Cartoon-to-real: An Approach to Translate Cartoon to Realistic Images using GAN
Nov 28, 2018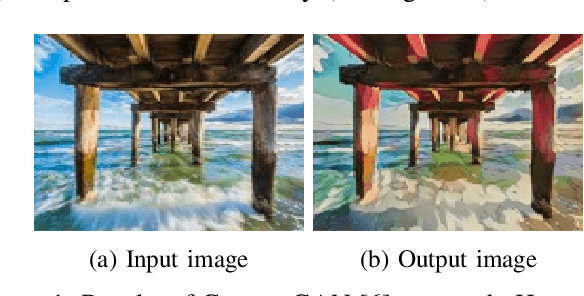

Abstract:We propose a method to translate cartoon images to real world images using Generative Aderserial Network (GAN). Existing GAN-based image-to-image translation methods which are trained on paired datasets are impractical as the data is difficult to accumulate. Therefore, in this paper we exploit the Cycle-Consistent Adversarial Networks (CycleGAN) method for images translation which needs an unpaired dataset. By applying CycleGAN we show that our model is able to generate meaningful real world images from cartoon images. However, we implement another state of the art technique $-$ Deep Analogy $-$ to compare the performance of our approach.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge