Zhidong Zhao
A Survey on Temporal Interaction Graph Representation Learning: Progress, Challenges, and Opportunities
May 07, 2025Abstract:Temporal interaction graphs (TIGs), defined by sequences of timestamped interaction events, have become ubiquitous in real-world applications due to their capability to model complex dynamic system behaviors. As a result, temporal interaction graph representation learning (TIGRL) has garnered significant attention in recent years. TIGRL aims to embed nodes in TIGs into low-dimensional representations that effectively preserve both structural and temporal information, thereby enhancing the performance of downstream tasks such as classification, prediction, and clustering within constantly evolving data environments. In this paper, we begin by introducing the foundational concepts of TIGs and emphasize the critical role of temporal dependencies. We then propose a comprehensive taxonomy of state-of-the-art TIGRL methods, systematically categorizing them based on the types of information utilized during the learning process to address the unique challenges inherent to TIGs. To facilitate further research and practical applications, we curate the source of datasets and benchmarks, providing valuable resources for empirical investigations. Finally, we examine key open challenges and explore promising research directions in TIGRL, laying the groundwork for future advancements that have the potential to shape the evolution of this field.
Knowledge-Guided Prompt Learning for Deepfake Facial Image Detection
Jan 01, 2025



Abstract:Recent generative models demonstrate impressive performance on synthesizing photographic images, which makes humans hardly to distinguish them from pristine ones, especially on realistic-looking synthetic facial images. Previous works mostly focus on mining discriminative artifacts from vast amount of visual data. However, they usually lack the exploration of prior knowledge and rarely pay attention to the domain shift between training categories (e.g., natural and indoor objects) and testing ones (e.g., fine-grained human facial images), resulting in unsatisfactory detection performance. To address these issues, we propose a novel knowledge-guided prompt learning method for deepfake facial image detection. Specifically, we retrieve forgery-related prompts from large language models as expert knowledge to guide the optimization of learnable prompts. Besides, we elaborate test-time prompt tuning to alleviate the domain shift, achieving significant performance improvement and boosting the application in real-world scenarios. Extensive experiments on DeepFakeFaceForensics dataset show that our proposed approach notably outperforms state-of-the-art methods.
Informative Subgraphs Aware Masked Auto-Encoder in Dynamic Graphs
Sep 14, 2024



Abstract:Generative self-supervised learning (SSL), especially masked autoencoders (MAE), has greatly succeeded and garnered substantial research interest in graph machine learning. However, the research of MAE in dynamic graphs is still scant. This gap is primarily due to the dynamic graph not only possessing topological structure information but also encapsulating temporal evolution dependency. Applying a random masking strategy which most MAE methods adopt to dynamic graphs will remove the crucial subgraph that guides the evolution of dynamic graphs, resulting in the loss of crucial spatio-temporal information in node representations. To bridge this gap, in this paper, we propose a novel Informative Subgraphs Aware Masked Auto-Encoder in Dynamic Graph, namely DyGIS. Specifically, we introduce a constrained probabilistic generative model to generate informative subgraphs that guide the evolution of dynamic graphs, successfully alleviating the issue of missing dynamic evolution subgraphs. The informative subgraph identified by DyGIS will serve as the input of dynamic graph masked autoencoder (DGMAE), effectively ensuring the integrity of the evolutionary spatio-temporal information within dynamic graphs. Extensive experiments on eleven datasets demonstrate that DyGIS achieves state-of-the-art performance across multiple tasks.
Accurate Segmentation of Optic Disc And Cup from Multiple Pseudo-labels by Noise-Aware Learning
Nov 30, 2023

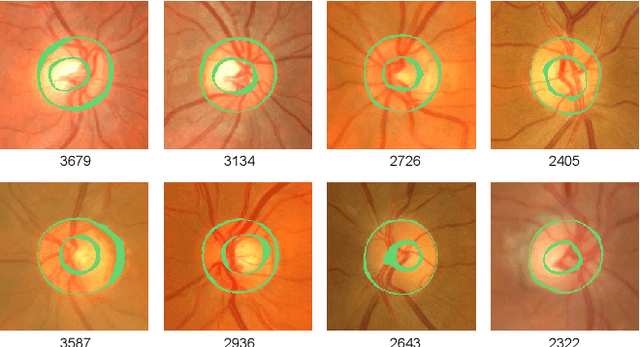

Abstract:Optic disc and cup segmentation play a crucial role in automating the screening and diagnosis of optic glaucoma. While data-driven convolutional neural networks (CNNs) show promise in this area, the inherent ambiguity of segmenting object and background boundaries in the task of optic disc and cup segmentation leads to noisy annotations that impact model performance. To address this, we propose an innovative label-denoising method of Multiple Pseudo-labels Noise-aware Network (MPNN) for accurate optic disc and cup segmentation. Specifically, the Multiple Pseudo-labels Generation and Guided Denoising (MPGGD) module generates pseudo-labels by multiple different initialization networks trained on true labels, and the pixel-level consensus information extracted from these pseudo-labels guides to differentiate clean pixels from noisy pixels. The training framework of the MPNN is constructed by a teacher-student architecture to learn segmentation from clean pixels and noisy pixels. Particularly, such a framework adeptly leverages (i) reliable and fundamental insights from clean pixels and (ii) the supplementary knowledge within noisy pixels via multiple perturbation-based unsupervised consistency. Compared to other label-denoising methods, comprehensive experimental results on the RIGA dataset demonstrate our method's excellent performance and significant denoising ability.
MSE-Nets: Multi-annotated Semi-supervised Ensemble Networks for Improving Segmentation of Medical Image with Ambiguous Boundaries
Nov 17, 2023



Abstract:Medical image segmentation annotations exhibit variations among experts due to the ambiguous boundaries of segmented objects and backgrounds in medical images. Although using multiple annotations for each image in the fully-supervised has been extensively studied for training deep models, obtaining a large amount of multi-annotated data is challenging due to the substantial time and manpower costs required for segmentation annotations, resulting in most images lacking any annotations. To address this, we propose Multi-annotated Semi-supervised Ensemble Networks (MSE-Nets) for learning segmentation from limited multi-annotated and abundant unannotated data. Specifically, we introduce the Network Pairwise Consistency Enhancement (NPCE) module and Multi-Network Pseudo Supervised (MNPS) module to enhance MSE-Nets for the segmentation task by considering two major factors: (1) to optimize the utilization of all accessible multi-annotated data, the NPCE separates (dis)agreement annotations of multi-annotated data at the pixel level and handles agreement and disagreement annotations in different ways, (2) to mitigate the introduction of imprecise pseudo-labels, the MNPS extends the training data by leveraging consistent pseudo-labels from unannotated data. Finally, we improve confidence calibration by averaging the predictions of base networks. Experiments on the ISIC dataset show that we reduced the demand for multi-annotated data by 97.75\% and narrowed the gap with the best fully-supervised baseline to just a Jaccard index of 4\%. Furthermore, compared to other semi-supervised methods that rely only on a single annotation or a combined fusion approach, the comprehensive experimental results on ISIC and RIGA datasets demonstrate the superior performance of our proposed method in medical image segmentation with ambiguous boundaries.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge