Zachary M. C. Baum
Biomechanics-informed Non-rigid Medical Image Registration and its Inverse Material Property Estimation with Linear and Nonlinear Elasticity
Jul 03, 2024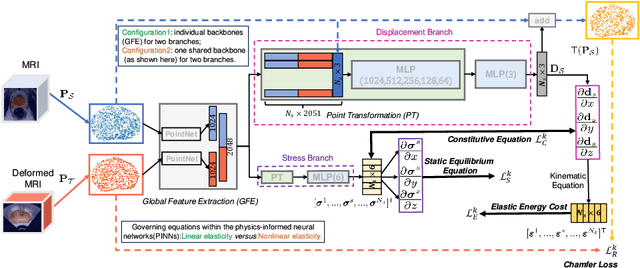
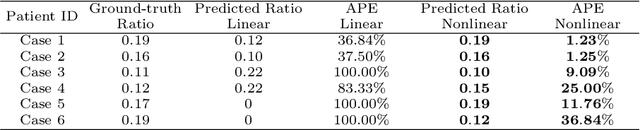
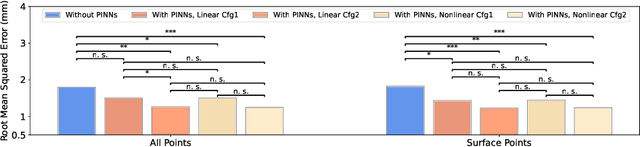
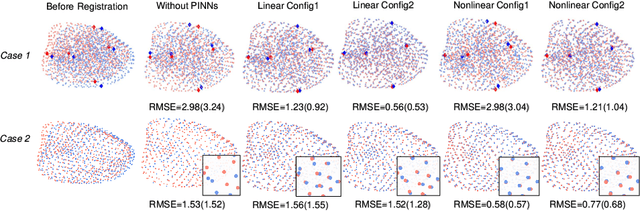
Abstract:This paper investigates both biomechanical-constrained non-rigid medical image registrations and accurate identifications of material properties for soft tissues, using physics-informed neural networks (PINNs). The complex nonlinear elasticity theory is leveraged to formally establish the partial differential equations (PDEs) representing physics laws of biomechanical constraints that need to be satisfied, with which registration and identification tasks are treated as forward (i.e., data-driven solutions of PDEs) and inverse (i.e., parameter estimation) problems under PINNs respectively. Two net configurations (i.e., Cfg1 and Cfg2) have also been compared for both linear and nonlinear physics model. Two sets of experiments have been conducted, using pairs of undeformed and deformed MR images from clinical cases of prostate cancer biopsy. Our contributions are summarised as follows. 1) We developed a learning-based biomechanical-constrained non-rigid registration algorithm using PINNs, where linear elasticity is generalised to the nonlinear version. 2) We demonstrated extensively that nonlinear elasticity shows no statistical significance against linear models in computing point-wise displacement vectors but their respective benefits may depend on specific patients, with finite-element (FE) computed ground-truth. 3) We formulated and solved the inverse parameter estimation problem, under the joint optimisation scheme of registration and parameter identification using PINNs, whose solutions can be accurately found by locating saddle points.
Boundary-RL: Reinforcement Learning for Weakly-Supervised Prostate Segmentation in TRUS Images
Aug 22, 2023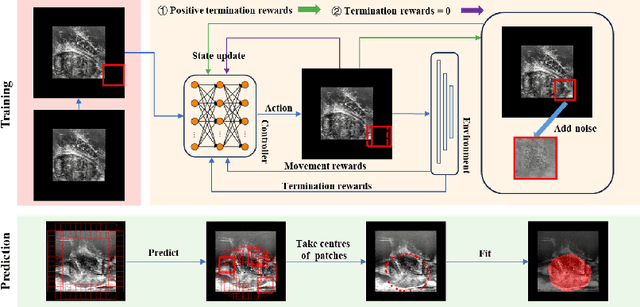
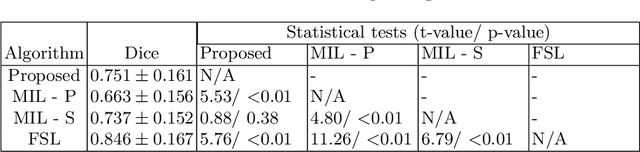
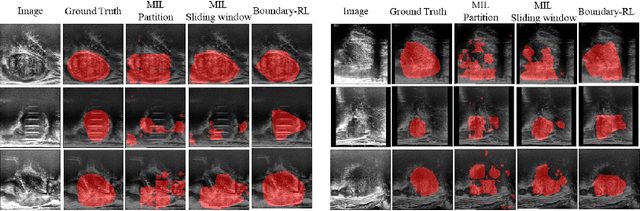
Abstract:We propose Boundary-RL, a novel weakly supervised segmentation method that utilises only patch-level labels for training. We envision the segmentation as a boundary detection problem, rather than a pixel-level classification as in previous works. This outlook on segmentation may allow for boundary delineation under challenging scenarios such as where noise artefacts may be present within the region-of-interest (ROI) boundaries, where traditional pixel-level classification-based weakly supervised methods may not be able to effectively segment the ROI. Particularly of interest, ultrasound images, where intensity values represent acoustic impedance differences between boundaries, may also benefit from the boundary delineation approach. Our method uses reinforcement learning to train a controller function to localise boundaries of ROIs using a reward derived from a pre-trained boundary-presence classifier. The classifier indicates when an object boundary is encountered within a patch, as the controller modifies the patch location in a sequential Markov decision process. The classifier itself is trained using only binary patch-level labels of object presence, which are the only labels used during training of the entire boundary delineation framework, and serves as a weak signal to inform the boundary delineation. The use of a controller function ensures that a sliding window over the entire image is not necessary. It also prevents possible false-positive or -negative cases by minimising number of patches passed to the boundary-presence classifier. We evaluate our proposed approach for a clinically relevant task of prostate gland segmentation on trans-rectal ultrasound images. We show improved performance compared to other tested weakly supervised methods, using the same labels e.g., multiple instance learning.
Non-rigid Medical Image Registration using Physics-informed Neural Networks
Feb 20, 2023



Abstract:Biomechanical modelling of soft tissue provides a non-data-driven method for constraining medical image registration, such that the estimated spatial transformation is considered biophysically plausible. This has not only been adopted in real-world clinical applications, such as the MR-to-ultrasound registration for prostate intervention of interest in this work, but also provides an explainable means of understanding the organ motion and spatial correspondence establishment. This work instantiates the recently-proposed physics-informed neural networks (PINNs) to a 3D linear elastic model for modelling prostate motion commonly encountered during transrectal ultrasound guided procedures. To overcome a widely-recognised challenge in generalising PINNs to different subjects, we propose to use PointNet as the nodal-permutation-invariant feature extractor, together with a registration algorithm that aligns point sets and simultaneously takes into account the PINN-imposed biomechanics. The proposed method has been both developed and validated in both patient-specific and multi-patient manner.
Meta-Learning Initializations for Interactive Medical Image Registration
Oct 27, 2022



Abstract:We present a meta-learning framework for interactive medical image registration. Our proposed framework comprises three components: a learning-based medical image registration algorithm, a form of user interaction that refines registration at inference, and a meta-learning protocol that learns a rapidly adaptable network initialization. This paper describes a specific algorithm that implements the registration, interaction and meta-learning protocol for our exemplar clinical application: registration of magnetic resonance (MR) imaging to interactively acquired, sparsely-sampled transrectal ultrasound (TRUS) images. Our approach obtains comparable registration error (4.26 mm) to the best-performing non-interactive learning-based 3D-to-3D method (3.97 mm) while requiring only a fraction of the data, and occurring in real-time during acquisition. Applying sparsely sampled data to non-interactive methods yields higher registration errors (6.26 mm), demonstrating the effectiveness of interactive MR-TRUS registration, which may be applied intraoperatively given the real-time nature of the adaptation process.
Image quality assessment for machine learning tasks using meta-reinforcement learning
Mar 27, 2022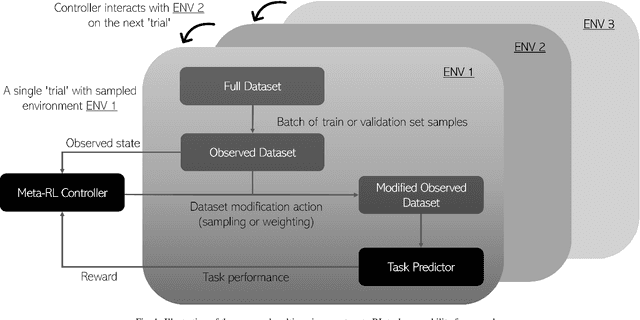
Abstract:In this paper, we consider image quality assessment (IQA) as a measure of how images are amenable with respect to a given downstream task, or task amenability. When the task is performed using machine learning algorithms, such as a neural-network-based task predictor for image classification or segmentation, the performance of the task predictor provides an objective estimate of task amenability. In this work, we use an IQA controller to predict the task amenability which, itself being parameterised by neural networks, can be trained simultaneously with the task predictor. We further develop a meta-reinforcement learning framework to improve the adaptability for both IQA controllers and task predictors, such that they can be fine-tuned efficiently on new datasets or meta-tasks. We demonstrate the efficacy of the proposed task-specific, adaptable IQA approach, using two clinical applications for ultrasound-guided prostate intervention and pneumonia detection on X-ray images.
* Accepted to Medical Image Analysis; Final published version available at: https://doi.org/10.1016/j.media.2022.102427
Image quality assessment by overlapping task-specific and task-agnostic measures: application to prostate multiparametric MR images for cancer segmentation
Feb 20, 2022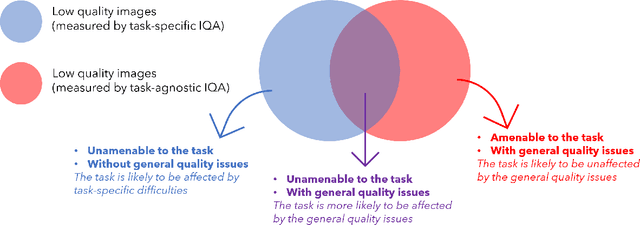
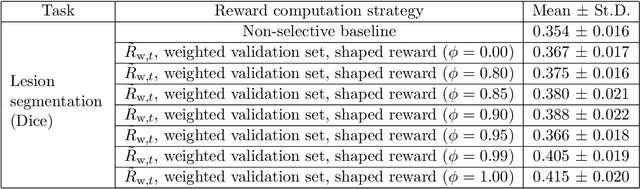

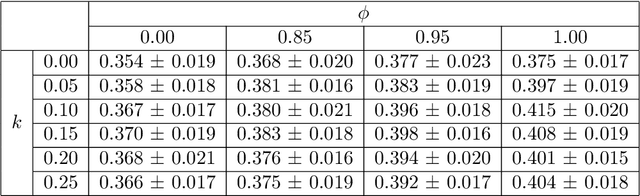
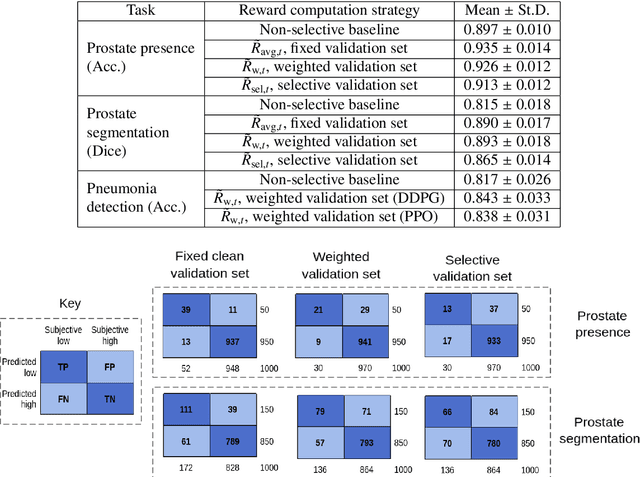
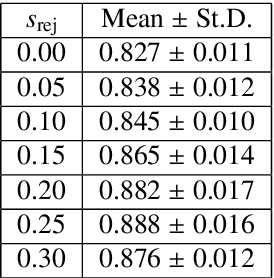
Abstract:Image quality assessment (IQA) in medical imaging can be used to ensure that downstream clinical tasks can be reliably performed. Quantifying the impact of an image on the specific target tasks, also named as task amenability, is needed. A task-specific IQA has recently been proposed to learn an image-amenability-predicting controller simultaneously with a target task predictor. This allows for the trained IQA controller to measure the impact an image has on the target task performance, when this task is performed using the predictor, e.g. segmentation and classification neural networks in modern clinical applications. In this work, we propose an extension to this task-specific IQA approach, by adding a task-agnostic IQA based on auto-encoding as the target task. Analysing the intersection between low-quality images, deemed by both the task-specific and task-agnostic IQA, may help to differentiate the underpinning factors that caused the poor target task performance. For example, common imaging artefacts may not adversely affect the target task, which would lead to a low task-agnostic quality and a high task-specific quality, whilst individual cases considered clinically challenging, which can not be improved by better imaging equipment or protocols, is likely to result in a high task-agnostic quality but a low task-specific quality. We first describe a flexible reward shaping strategy which allows for the adjustment of weighting between task-agnostic and task-specific quality scoring. Furthermore, we evaluate the proposed algorithm using a clinically challenging target task of prostate tumour segmentation on multiparametric magnetic resonance (mpMR) images, from 850 patients. The proposed reward shaping strategy, with appropriately weighted task-specific and task-agnostic qualities, successfully identified samples that need re-acquisition due to defected imaging process.
Adaptable image quality assessment using meta-reinforcement learning of task amenability
Jul 31, 2021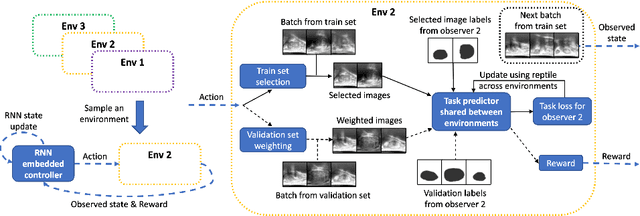
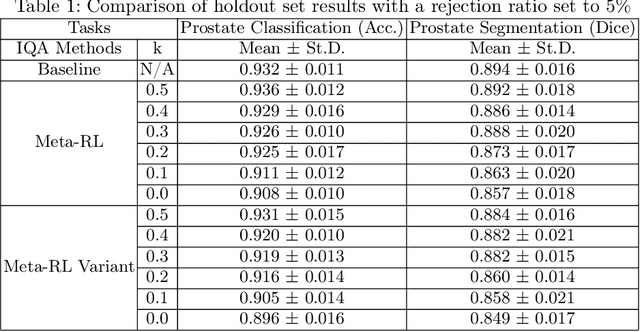
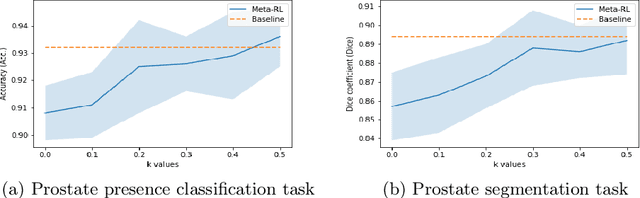
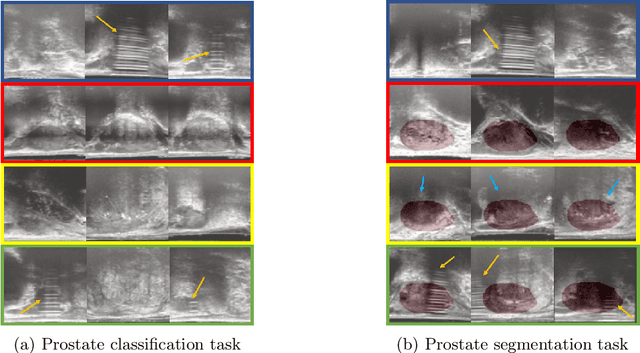
Abstract:The performance of many medical image analysis tasks are strongly associated with image data quality. When developing modern deep learning algorithms, rather than relying on subjective (human-based) image quality assessment (IQA), task amenability potentially provides an objective measure of task-specific image quality. To predict task amenability, an IQA agent is trained using reinforcement learning (RL) with a simultaneously optimised task predictor, such as a classification or segmentation neural network. In this work, we develop transfer learning or adaptation strategies to increase the adaptability of both the IQA agent and the task predictor so that they are less dependent on high-quality, expert-labelled training data. The proposed transfer learning strategy re-formulates the original RL problem for task amenability in a meta-reinforcement learning (meta-RL) framework. The resulting algorithm facilitates efficient adaptation of the agent to different definitions of image quality, each with its own Markov decision process environment including different images, labels and an adaptable task predictor. Our work demonstrates that the IQA agents pre-trained on non-expert task labels can be adapted to predict task amenability as defined by expert task labels, using only a small set of expert labels. Using 6644 clinical ultrasound images from 249 prostate cancer patients, our results for image classification and segmentation tasks show that the proposed IQA method can be adapted using data with as few as respective 19.7% and 29.6% expert-reviewed consensus labels and still achieve comparable IQA and task performance, which would otherwise require a training dataset with 100% expert labels.
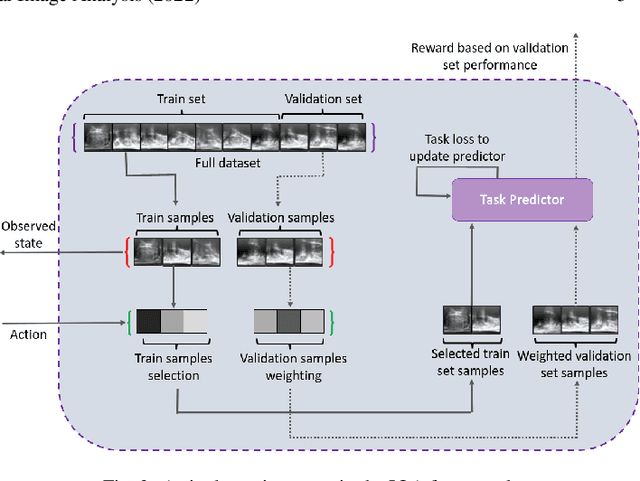
Learning image quality assessment by reinforcing task amenable data selection
Feb 15, 2021



Abstract:In this paper, we consider a type of image quality assessment as a task-specific measurement, which can be used to select images that are more amenable to a given target task, such as image classification or segmentation. We propose to train simultaneously two neural networks for image selection and a target task using reinforcement learning. A controller network learns an image selection policy by maximising an accumulated reward based on the target task performance on the controller-selected validation set, whilst the target task predictor is optimised using the training set. The trained controller is therefore able to reject those images that lead to poor accuracy in the target task. In this work, we show that the controller-predicted image quality can be significantly different from the task-specific image quality labels that are manually defined by humans. Furthermore, we demonstrate that it is possible to learn effective image quality assessment without using a ``clean'' validation set, thereby avoiding the requirement for human labelling of images with respect to their amenability for the task. Using $6712$, labelled and segmented, clinical ultrasound images from $259$ patients, experimental results on holdout data show that the proposed image quality assessment achieved a mean classification accuracy of $0.94\pm0.01$ and a mean segmentation Dice of $0.89\pm0.02$, by discarding $5\%$ and $15\%$ of the acquired images, respectively. The significantly improved performance was observed for both tested tasks, compared with the respective $0.90\pm0.01$ and $0.82\pm0.02$ from networks without considering task amenability. This enables image quality feedback during real-time ultrasound acquisition among many other medical imaging applications.
DeepReg: a deep learning toolkit for medical image registration
Nov 04, 2020Abstract:DeepReg (https://github.com/DeepRegNet/DeepReg) is a community-supported open-source toolkit for research and education in medical image registration using deep learning.
Multimodality Biomedical Image Registration using Free Point Transformer Networks
Aug 05, 2020



Abstract:We describe a point-set registration algorithm based on a novel free point transformer (FPT) network, designed for points extracted from multimodal biomedical images for registration tasks, such as those frequently encountered in ultrasound-guided interventional procedures. FPT is constructed with a global feature extractor which accepts unordered source and target point-sets of variable size. The extracted features are conditioned by a shared multilayer perceptron point transformer module to predict a displacement vector for each source point, transforming it into the target space. The point transformer module assumes no vicinity or smoothness in predicting spatial transformation and, together with the global feature extractor, is trained in a data-driven fashion with an unsupervised loss function. In a multimodal registration task using prostate MR and sparsely acquired ultrasound images, FPT yields comparable or improved results over other rigid and non-rigid registration methods. This demonstrates the versatility of FPT to learn registration directly from real, clinical training data and to generalize to a challenging task, such as the interventional application presented.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge