Weixi Yi
T2-Only Prostate Cancer Prediction by Meta-Learning from Bi-Parametric MR Imaging
Nov 11, 2024


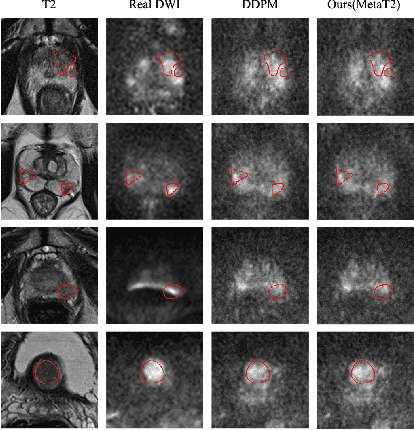
Abstract:Current imaging-based prostate cancer diagnosis requires both MR T2-weighted (T2w) and diffusion-weighted imaging (DWI) sequences, with additional sequences for potentially greater accuracy improvement. However, measuring diffusion patterns in DWI sequences can be time-consuming, prone to artifacts and sensitive to imaging parameters. While machine learning (ML) models have demonstrated radiologist-level accuracy in detecting prostate cancer from these two sequences, this study investigates the potential of ML-enabled methods using only the T2w sequence as input during inference time. We first discuss the technical feasibility of such a T2-only approach, and then propose a novel ML formulation, where DWI sequences - readily available for training purposes - are only used to train a meta-learning model, which subsequently only uses T2w sequences at inference. Using multiple datasets from more than 3,000 prostate cancer patients, we report superior or comparable performance in localising radiologist-identified prostate cancer using our proposed T2-only models, compared with alternative models using T2-only or both sequences as input. Real patient cases are presented and discussed to demonstrate, for the first time, the exclusively true-positive cases from models with different input sequences.
Boundary-RL: Reinforcement Learning for Weakly-Supervised Prostate Segmentation in TRUS Images
Aug 22, 2023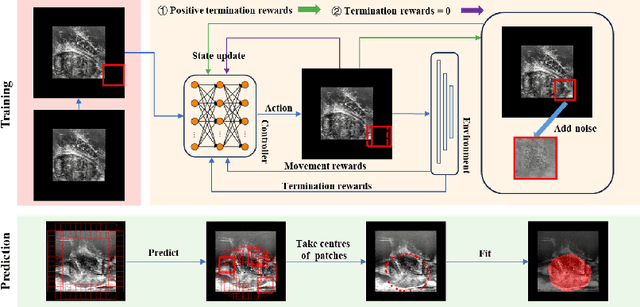
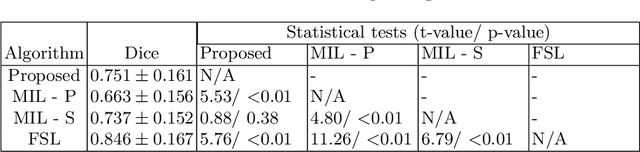
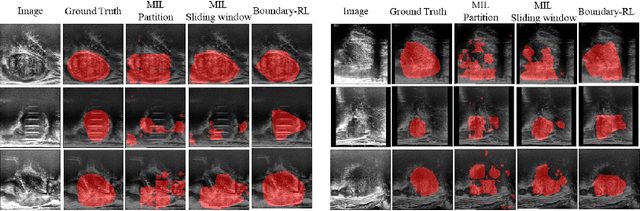
Abstract:We propose Boundary-RL, a novel weakly supervised segmentation method that utilises only patch-level labels for training. We envision the segmentation as a boundary detection problem, rather than a pixel-level classification as in previous works. This outlook on segmentation may allow for boundary delineation under challenging scenarios such as where noise artefacts may be present within the region-of-interest (ROI) boundaries, where traditional pixel-level classification-based weakly supervised methods may not be able to effectively segment the ROI. Particularly of interest, ultrasound images, where intensity values represent acoustic impedance differences between boundaries, may also benefit from the boundary delineation approach. Our method uses reinforcement learning to train a controller function to localise boundaries of ROIs using a reward derived from a pre-trained boundary-presence classifier. The classifier indicates when an object boundary is encountered within a patch, as the controller modifies the patch location in a sequential Markov decision process. The classifier itself is trained using only binary patch-level labels of object presence, which are the only labels used during training of the entire boundary delineation framework, and serves as a weak signal to inform the boundary delineation. The use of a controller function ensures that a sliding window over the entire image is not necessary. It also prevents possible false-positive or -negative cases by minimising number of patches passed to the boundary-presence classifier. We evaluate our proposed approach for a clinically relevant task of prostate gland segmentation on trans-rectal ultrasound images. We show improved performance compared to other tested weakly supervised methods, using the same labels e.g., multiple instance learning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge