Youssef Skandarani
Deep Learning methods for automatic evaluation of delayed enhancement-MRI. The results of the EMIDEC challenge
Aug 10, 2021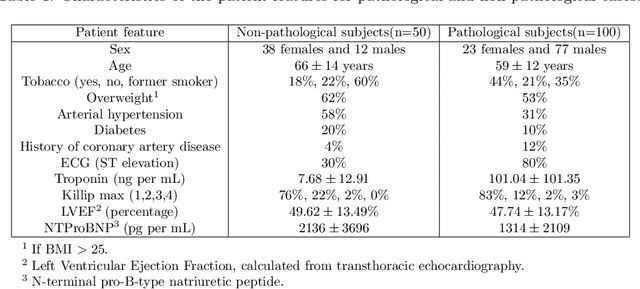
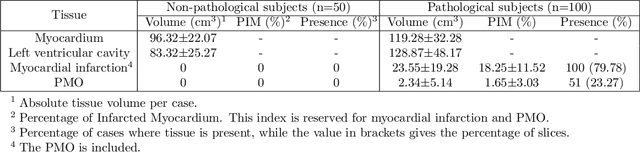
Abstract:A key factor for assessing the state of the heart after myocardial infarction (MI) is to measure whether the myocardium segment is viable after reperfusion or revascularization therapy. Delayed enhancement-MRI or DE-MRI, which is performed several minutes after injection of the contrast agent, provides high contrast between viable and nonviable myocardium and is therefore a method of choice to evaluate the extent of MI. To automatically assess myocardial status, the results of the EMIDEC challenge that focused on this task are presented in this paper. The challenge's main objectives were twofold. First, to evaluate if deep learning methods can distinguish between normal and pathological cases. Second, to automatically calculate the extent of myocardial infarction. The publicly available database consists of 150 exams divided into 50 cases with normal MRI after injection of a contrast agent and 100 cases with myocardial infarction (and then with a hyperenhanced area on DE-MRI), whatever their inclusion in the cardiac emergency department. Along with MRI, clinical characteristics are also provided. The obtained results issued from several works show that the automatic classification of an exam is a reachable task (the best method providing an accuracy of 0.92), and the automatic segmentation of the myocardium is possible. However, the segmentation of the diseased area needs to be improved, mainly due to the small size of these areas and the lack of contrast with the surrounding structures.
Deep Learning Based Cardiac MRI Segmentation: Do We Need Experts?
Jul 23, 2021



Abstract:Deep learning methods are the de-facto solutions to a multitude of medical image analysis tasks. Cardiac MRI segmentation is one such application which, like many others, requires a large number of annotated data so a trained network can generalize well. Unfortunately, the process of having a large number of manually curated images by medical experts is both slow and utterly expensive. In this paper, we set out to explore whether expert knowledge is a strict requirement for the creation of annotated datasets that machine learning can successfully train on. To do so, we gauged the performance of three segmentation models, namely U-Net, Attention U-Net, and ENet, trained with different loss functions on expert and non-expert groundtruth for cardiac cine-MRI segmentation. Evaluation was done with classic segmentation metrics (Dice index and Hausdorff distance) as well as clinical measurements, such as the ventricular ejection fractions and the myocardial mass. Results reveal that generalization performances of a segmentation neural network trained on non-expert groundtruth data is, to all practical purposes, as good as on expert groundtruth data, in particular when the non-expert gets a decent level of training, highlighting an opportunity for the efficient and cheap creation of annotations for cardiac datasets.
GANs for Medical Image Synthesis: An Empirical Study
May 11, 2021



Abstract:Generative Adversarial Networks (GANs) have become increasingly powerful, generating mind-blowing photorealistic images that mimic the content of datasets they were trained to replicate. One recurrent theme in medical imaging is whether GANs can also be effective at generating workable medical data as they are for generating realistic RGB images. In this paper, we perform a multi-GAN and multi-application study to gauge the benefits of GANs in medical imaging. We tested various GAN architectures from basic DCGAN to more sophisticated style-based GANs on three medical imaging modalities and organs namely : cardiac cine-MRI, liver CT and RGB retina images. GANs were trained on well-known and widely utilized datasets from which their FID score were computed to measure the visual acuity of their generated images. We further tested their usefulness by measuring the segmentation accuracy of a U-Net trained on these generated images. Results reveal that GANs are far from being equal as some are ill-suited for medical imaging applications while others are much better off. The top-performing GANs are capable of generating realistic-looking medical images by FID standards that can fool trained experts in a visual Turing test and comply to some metrics. However, segmentation results suggests that no GAN is capable of reproducing the full richness of a medical datasets.
Neural Teleportation
Dec 02, 2020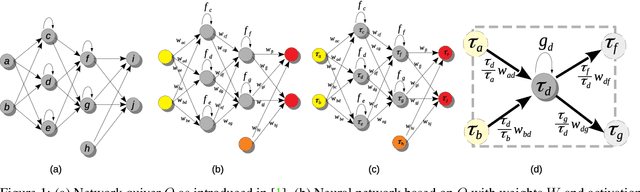
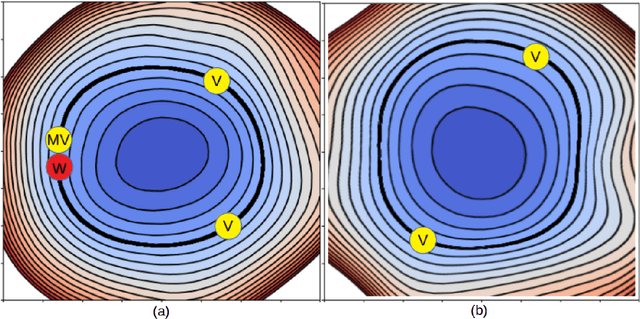
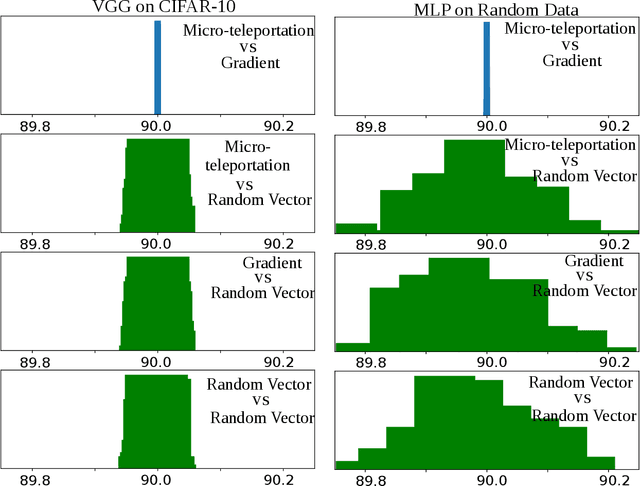
Abstract:In this paper, we explore a process called neural teleportation, a mathematical consequence of applying quiver representation theory to neural networks. Neural teleportation "teleports" a network to a new position in the weight space, while leaving its function unchanged. This concept generalizes the notion of positive scale invariance of ReLU networks to any network with any activation functions and any architecture. In this paper, we shed light on surprising and counter-intuitive consequences neural teleportation has on the loss landscape. In particular, we show that teleportation can be used to explore loss level curves, that it changes the loss landscape, sharpens global minima and boosts back-propagated gradients. From these observations, we demonstrate that teleportation accelerates training when used during initialization regardless of the model, its activation function, the loss function, and the training data. Our results can be reproduced with the code available here: https://github.com/vitalab/neuralteleportation.
Automatic Myocardial Infarction Evaluation from Delayed-Enhancement Cardiac MRI using Deep Convolutional Networks
Oct 30, 2020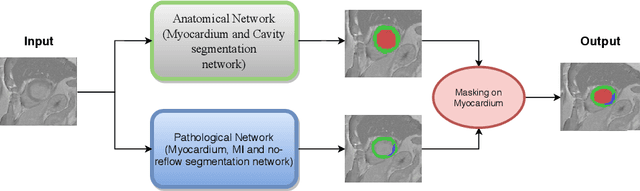

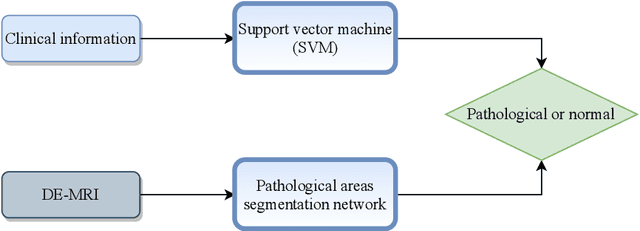
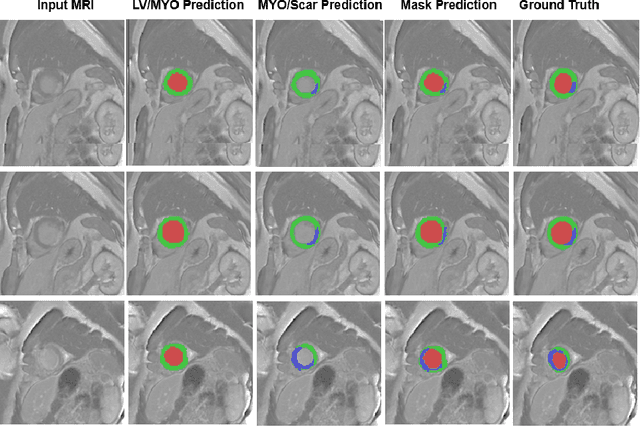
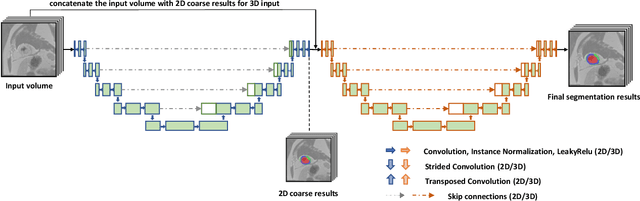
Abstract:In this paper, we propose a new deep learning framework for an automatic myocardial infarction evaluation from clinical information and delayed enhancement-MRI (DE-MRI). The proposed framework addresses two tasks. The first task is automatic detection of myocardial contours, the infarcted area, the no-reflow area, and the left ventricular cavity from a short-axis DE-MRI series. It employs two segmentation neural networks. The first network is used to segment the anatomical structures such as the myocardium and left ventricular cavity. The second network is used to segment the pathological areas such as myocardial infarction, myocardial no-reflow, and normal myocardial region. The segmented myocardium region from the first network is further used to refine the second network's pathological segmentation results. The second task is to automatically classify a given case into normal or pathological from clinical information with or without DE-MRI. A cascaded support vector machine (SVM) is employed to classify a given case from its associated clinical information. The segmented pathological areas from DE-MRI are also used for the classification task. We evaluated our method on the 2020 EMIDEC MICCAI challenge dataset. It yielded an average Dice index of 0.93 and 0.84, respectively, for the left ventricular cavity and the myocardium. The classification from using only clinical information yielded 80% accuracy over five-fold cross-validation. Using the DE-MRI, our method can classify the cases with 93.3% accuracy. These experimental results reveal that the proposed method can automatically evaluate the myocardial infarction.
Cardiac Segmentation with Strong Anatomical Guarantees
Jun 15, 2020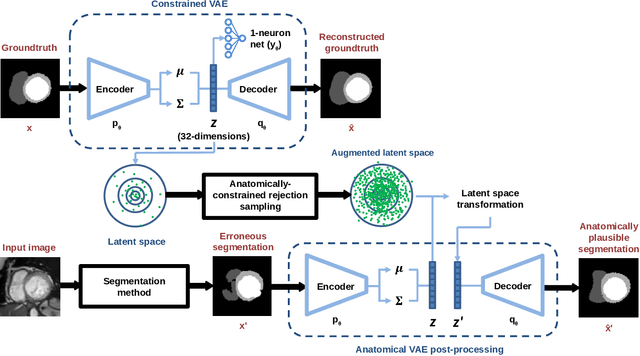
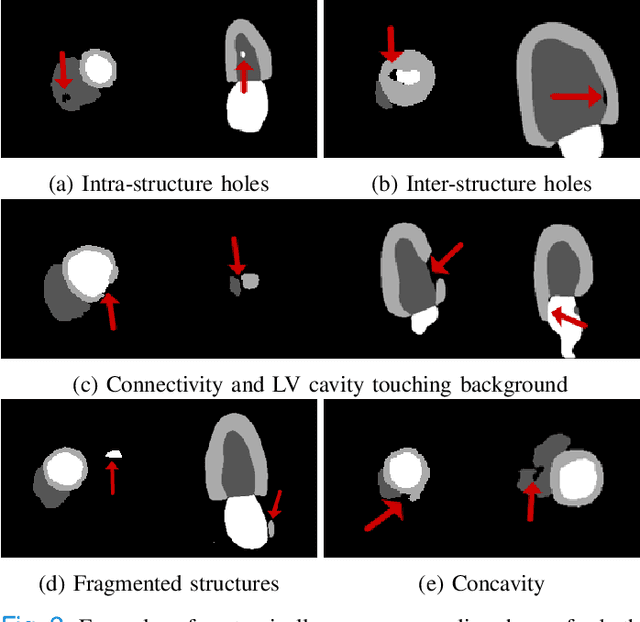
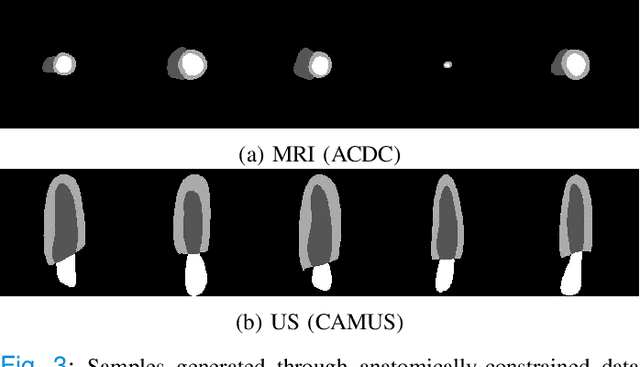
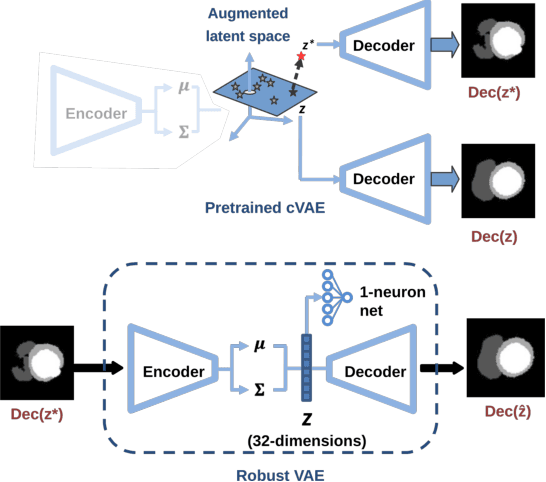
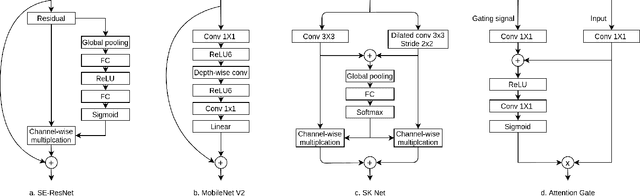
Abstract:Convolutional neural networks (CNN) have had unprecedented success in medical imaging and, in particular, in medical image segmentation. However, despite the fact that segmentation results are closer than ever to the inter-expert variability, CNNs are not immune to producing anatomically inaccurate segmentations, even when built upon a shape prior. In this paper, we present a framework for producing cardiac image segmentation maps that are guaranteed to respect pre-defined anatomical criteria, while remaining within the inter-expert variability. The idea behind our method is to use a well-trained CNN, have it process cardiac images, identify the anatomically implausible results and warp these results toward the closest anatomically valid cardiac shape. This warping procedure is carried out with a constrained variational autoencoder (cVAE) trained to learn a representation of valid cardiac shapes through a smooth, yet constrained, latent space. With this cVAE, we can project any implausible shape into the cardiac latent space and steer it toward the closest correct shape. We tested our framework on short-axis MRI as well as apical two and four-chamber view ultrasound images, two modalities for which cardiac shapes are drastically different. With our method, CNNs can now produce results that are both within the inter-expert variability and always anatomically plausible without having to rely on a shape prior.
On the effectiveness of GAN generated cardiac MRIs for segmentation
May 22, 2020

Abstract:In this work, we propose a Variational Autoencoder (VAE) - Generative Adversarial Networks (GAN) model that can produce highly realistic MRI together with its pixel accurate groundtruth for the application of cine-MR image cardiac segmentation. On one side of our model is a Variational Autoencoder (VAE) trained to learn the latent representations of cardiac shapes. On the other side is a GAN that uses "SPatially-Adaptive (DE)Normalization" (SPADE) modules to generate realistic MR images tailored to a given anatomical map. At test time, the sampling of the VAE latent space allows to generate an arbitrary large number of cardiac shapes, which are fed to the GAN that subsequently generates MR images whose cardiac structure fits that of the cardiac shapes. In other words, our system can generate a large volume of realistic yet labeled cardiac MR images. We show that segmentation with CNNs trained with our synthetic annotated images gets competitive results compared to traditional techniques. We also show that combining data augmentation with our GAN-generated images lead to an improvement in the Dice score of up to 12 percent while allowing for better generalization capabilities on other datasets.
Cardiac MRI Segmentation with Strong Anatomical Guarantees
Jul 05, 2019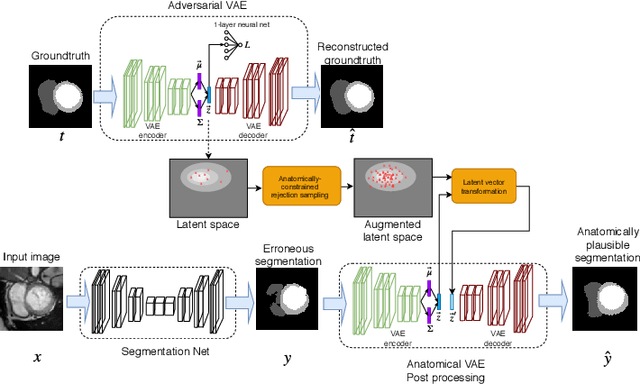
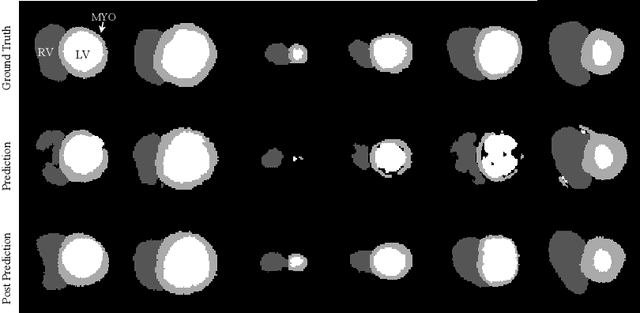
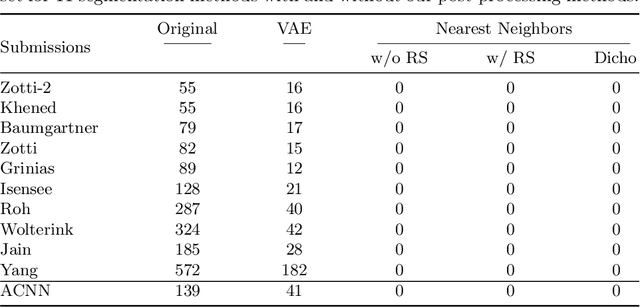
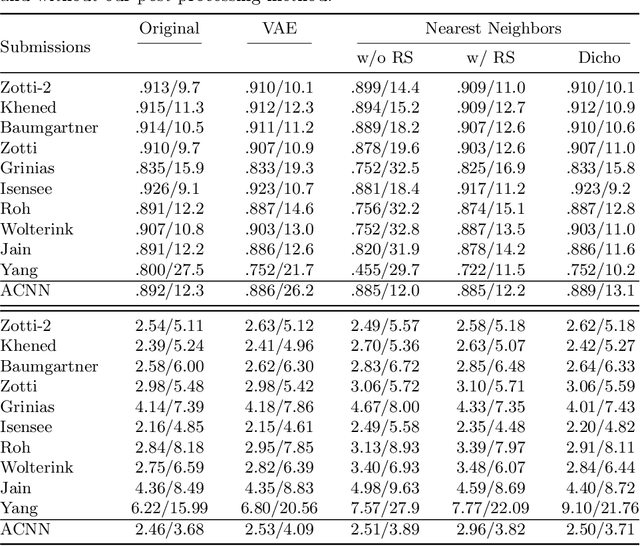
Abstract:Recent publications have shown that the segmentation accuracy of modern-day convolutional neural networks (CNN) applied on cardiac MRI can reach the inter-expert variability, a great achievement in this area of research. However, despite these successes, CNNs still produce anatomically inaccurate segmentations as they provide no guarantee on the anatomical plausibility of their outcome, even when using a shape prior. In this paper, we propose a cardiac MRI segmentation method which always produces anatomically plausible results. At the core of the method is an adversarial variational autoencoder (aVAE) whose latent space encodes a smooth manifold on which lies a large spectrum of valid cardiac shapes. This aVAE is used to automatically warp anatomically inaccurate cardiac shapes towards a close but correct shape. Our method can accommodate any cardiac segmentation method and convert its anatomically implausible results to plausible ones without affecting its overall geometric and clinical metrics. With our method, CNNs can now produce results that are both within the inter-expert variability and always anatomically plausible.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge