Thomas Decourselle
Deep Learning methods for automatic evaluation of delayed enhancement-MRI. The results of the EMIDEC challenge
Aug 10, 2021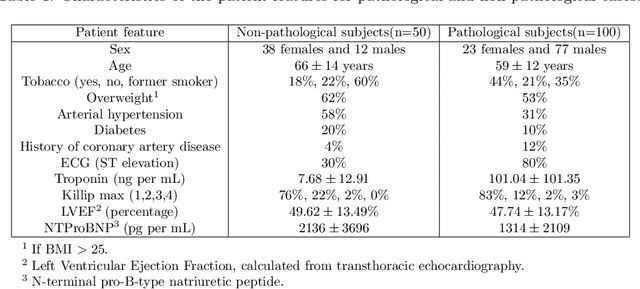
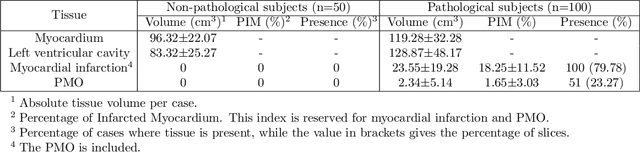
Abstract:A key factor for assessing the state of the heart after myocardial infarction (MI) is to measure whether the myocardium segment is viable after reperfusion or revascularization therapy. Delayed enhancement-MRI or DE-MRI, which is performed several minutes after injection of the contrast agent, provides high contrast between viable and nonviable myocardium and is therefore a method of choice to evaluate the extent of MI. To automatically assess myocardial status, the results of the EMIDEC challenge that focused on this task are presented in this paper. The challenge's main objectives were twofold. First, to evaluate if deep learning methods can distinguish between normal and pathological cases. Second, to automatically calculate the extent of myocardial infarction. The publicly available database consists of 150 exams divided into 50 cases with normal MRI after injection of a contrast agent and 100 cases with myocardial infarction (and then with a hyperenhanced area on DE-MRI), whatever their inclusion in the cardiac emergency department. Along with MRI, clinical characteristics are also provided. The obtained results issued from several works show that the automatic classification of an exam is a reachable task (the best method providing an accuracy of 0.92), and the automatic segmentation of the myocardium is possible. However, the segmentation of the diseased area needs to be improved, mainly due to the small size of these areas and the lack of contrast with the surrounding structures.
Segmentation of the Myocardium on Late-Gadolinium Enhanced MRI based on 2.5 D Residual Squeeze and Excitation Deep Learning Model
May 27, 2020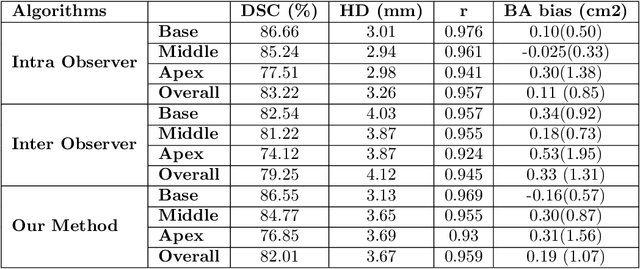
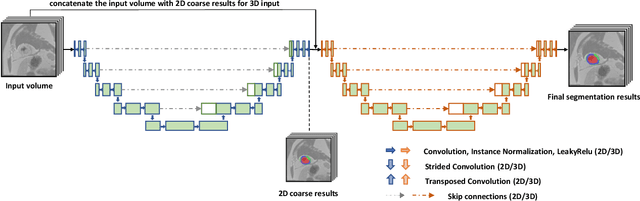
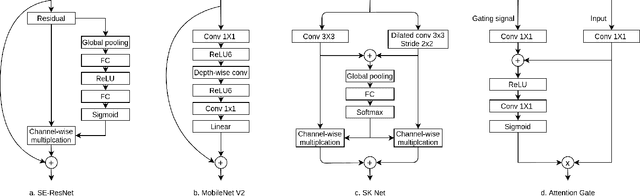
Abstract:Cardiac left ventricular (LV) segmentation from short-axis MRI acquired 10 minutes after the injection of a contrast agent (LGE-MRI) is a necessary step in the processing allowing the identification and diagnosis of cardiac diseases such as myocardial infarction. However, this segmentation is challenging due to high variability across subjects and the potential lack of contrast between structures. Then, the main objective of this work is to develop an accurate automatic segmentation method based on deep learning models for the myocardial borders on LGE-MRI. To this end, 2.5 D residual neural network integrated with a squeeze and excitation blocks in encoder side with specialized convolutional has been proposed. Late fusion has been used to merge the output of the best trained proposed models from a different set of hyperparameters. A total number of 320 exams (with a mean number of 6 slices per exam) were used for training and 28 exams used for testing. The performance analysis of the proposed ensemble model in the basal and middle slices was similar as compared to intra-observer study and slightly lower at apical slices. The overall Dice score was 82.01% by our proposed method as compared to Dice score of 83.22% obtained from the intra observer study. The proposed model could be used for the automatic segmentation of myocardial border that is a very important step for accurate quantification of no-reflow, myocardial infarction, myocarditis, and hypertrophic cardiomyopathy, among others.
Myocardial Infarction Quantification From Late Gadolinium Enhancement MRI Using Top-hat Transforms and Neural Networks
Jan 09, 2019



Abstract:Significance: Late gadolinium enhanced magnetic resonance imaging (LGE-MRI) is the gold standard technique for myocardial viability assessment. Although the technique accurately reflects the damaged tissue, there is no clinical standard for quantifying myocardial infarction (MI), demanding most algorithms to be expert dependent. Objectives and Methods: In this work a new automatic method for MI quantification from LGE-MRI is proposed. Our novel segmentation approach is devised for accurately detecting not only hyper-enhanced lesions, but also microvascular-obstructed areas. Moreover, it includes a myocardial disease detection step which extends the algorithm for working under healthy scans. The method is based on a cascade approach where firstly, diseased slices are identified by a convolutional neural network (CNN). Secondly, by means of morphological operations a fast coarse scar segmentation is obtained. Thirdly, the segmentation is refined by a boundary-voxel reclassification strategy using an ensemble of CNNs. For its validation, reproducibility and further comparison against other methods, we tested the method on a big multi-field expert annotated LGE-MRI database including healthy and diseased cases. Results and Conclusion: In an exhaustive comparison against nine reference algorithms, the proposal achieved state-of-the-art segmentation performances and showed to be the only method agreeing in volumetric scar quantification with the expert delineations. Moreover, the method was able to reproduce the intra- and inter-observer variability ranges. It is concluded that the method could suitably be transferred to clinical scenarios.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge