Yongyi Shi
Zero-Shot Low-dose CT Denoising via Sinogram Flicking
Apr 10, 2025Abstract:Many low-dose CT imaging methods rely on supervised learning, which requires a large number of paired noisy and clean images. However, obtaining paired images in clinical practice is challenging. To address this issue, zero-shot self-supervised methods train denoising networks using only the information within a single image, such as ZS-N2N. However, these methods often employ downsampling operations that degrade image resolution. Additionally, the training dataset is inherently constrained to the image itself. In this paper, we propose a zero-shot low-dose CT imaging method based on sinogram flicking, which operates within a single image but generates many copies via random conjugate ray matching. Specifically, two conjugate X-ray pencil beams measure the same path; their expected values should be identical, while their noise levels vary during measurements. By randomly swapping portions of the conjugate X-rays in the sinogram domain, we generate a large set of sinograms with consistent content but varying noise patterns. When displayed dynamically, these sinograms exhibit a flickering effect due to their identical structural content but differing noise patterns-hence the term sinogram flicking. We train the network on pairs of sinograms with the same content but different noise distributions using a lightweight model adapted from ZS-NSN. This process is repeated to obtain the final results. A simulation study demonstrates that our method outperforms state-of-the-art approaches such as ZS-N2N.
Few-Shot Generation of Brain Tumors for Secure and Fair Data Sharing
Mar 31, 2025Abstract:Leveraging multi-center data for medical analytics presents challenges due to privacy concerns and data heterogeneity. While distributed approaches such as federated learning has gained traction, they remain vulnerable to privacy breaches, particularly in sensitive domains like medical imaging. Generative models, such as diffusion models, enhance privacy by synthesizing realistic data. However, they are prone to memorization, especially when trained on small datasets. This study proposes a decentralized few-shot generative model (DFGM) to synthesize brain tumor images while fully preserving privacy. DFGM harmonizes private tumor data with publicly shareable healthy images from multiple medical centers, constructing a new dataset by blending tumor foregrounds with healthy backgrounds. This approach ensures stringent privacy protection and enables controllable, high-quality synthesis by preserving both the healthy backgrounds and tumor foregrounds. We assess DFGM's effectiveness in brain tumor segmentation using a UNet, achieving Dice score improvements of 3.9% for data augmentation and 4.6% for fairness on a separate dataset.
Diffusion Prior Regularized Iterative Reconstruction for Low-dose CT
Oct 10, 2023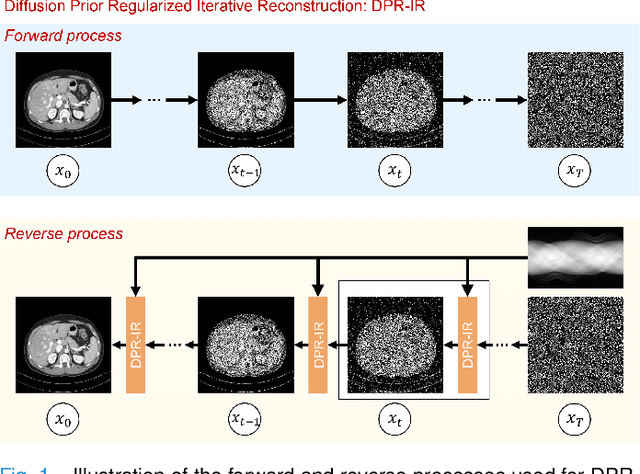
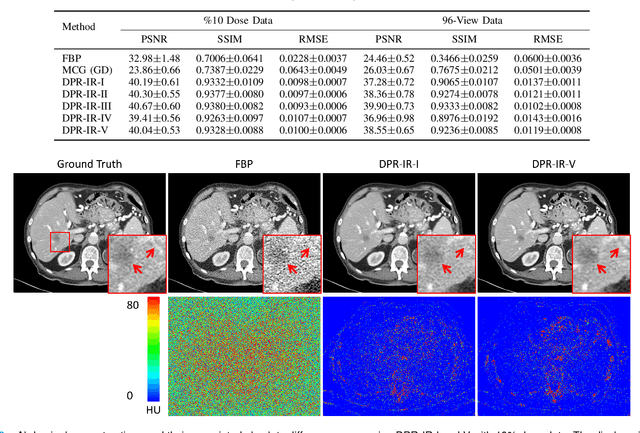
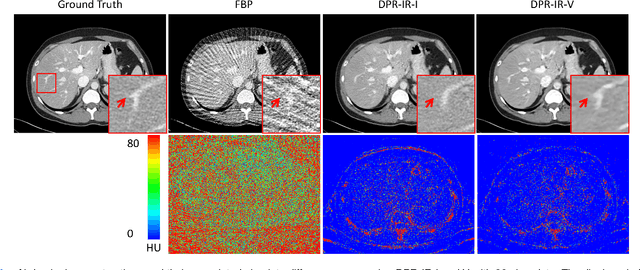
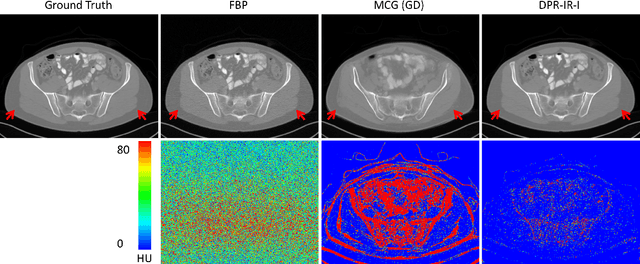
Abstract:Computed tomography (CT) involves a patient's exposure to ionizing radiation. To reduce the radiation dose, we can either lower the X-ray photon count or down-sample projection views. However, either of the ways often compromises image quality. To address this challenge, here we introduce an iterative reconstruction algorithm regularized by a diffusion prior. Drawing on the exceptional imaging prowess of the denoising diffusion probabilistic model (DDPM), we merge it with a reconstruction procedure that prioritizes data fidelity. This fusion capitalizes on the merits of both techniques, delivering exceptional reconstruction results in an unsupervised framework. To further enhance the efficiency of the reconstruction process, we incorporate the Nesterov momentum acceleration technique. This enhancement facilitates superior diffusion sampling in fewer steps. As demonstrated in our experiments, our method offers a potential pathway to high-definition CT image reconstruction with minimized radiation.
Blind CT Image Quality Assessment Using DDPM-derived Content and Transformer-based Evaluator
Oct 04, 2023Abstract:Lowering radiation dose per view and utilizing sparse views per scan are two common CT scan modes, albeit often leading to distorted images characterized by noise and streak artifacts. Blind image quality assessment (BIQA) strives to evaluate perceptual quality in alignment with what radiologists perceive, which plays an important role in advancing low-dose CT reconstruction techniques. An intriguing direction involves developing BIQA methods that mimic the operational characteristic of the human visual system (HVS). The internal generative mechanism (IGM) theory reveals that the HVS actively deduces primary content to enhance comprehension. In this study, we introduce an innovative BIQA metric that emulates the active inference process of IGM. Initially, an active inference module, implemented as a denoising diffusion probabilistic model (DDPM), is constructed to anticipate the primary content. Then, the dissimilarity map is derived by assessing the interrelation between the distorted image and its primary content. Subsequently, the distorted image and dissimilarity map are combined into a multi-channel image, which is inputted into a transformer-based image quality evaluator. Remarkably, by exclusively utilizing this transformer-based quality evaluator, we won the second place in the MICCAI 2023 low-dose computed tomography perceptual image quality assessment grand challenge. Leveraging the DDPM-derived primary content, our approach further improves the performance on the challenge dataset.
Lesion classification by model-based feature extraction: A differential affine invariant model of soft tissue elasticity
May 27, 2022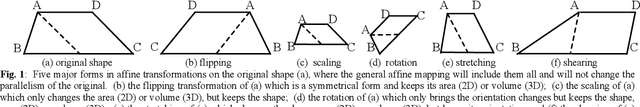
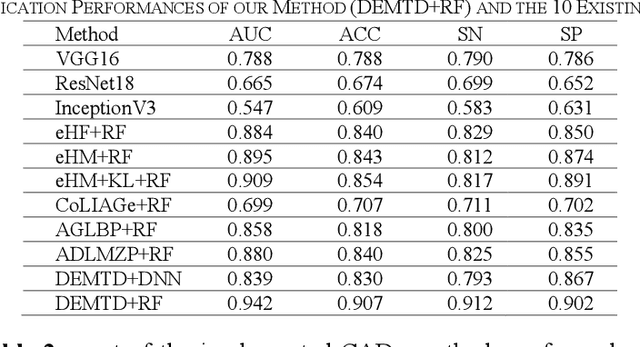
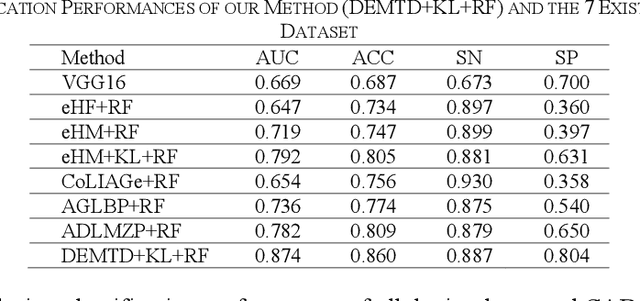
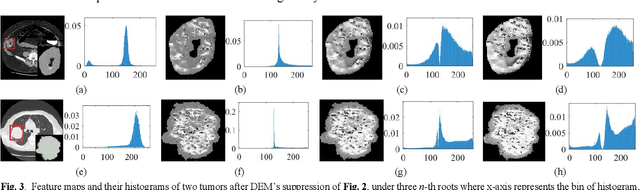
Abstract:The elasticity of soft tissues has been widely considered as a characteristic property to differentiate between healthy and vicious tissues and, therefore, motivated several elasticity imaging modalities, such as Ultrasound Elastography, Magnetic Resonance Elastography, and Optical Coherence Elastography. This paper proposes an alternative approach of modeling the elasticity using Computed Tomography (CT) imaging modality for model-based feature extraction machine learning (ML) differentiation of lesions. The model describes a dynamic non-rigid (or elastic) deformation in differential manifold to mimic the soft tissues elasticity under wave fluctuation in vivo. Based on the model, three local deformation invariants are constructed by two tensors defined by the first and second order derivatives from the CT images and used to generate elastic feature maps after normalization via a novel signal suppression method. The model-based elastic image features are extracted from the feature maps and fed to machine learning to perform lesion classifications. Two pathologically proven image datasets of colon polyps (44 malignant and 43 benign) and lung nodules (46 malignant and 20 benign) were used to evaluate the proposed model-based lesion classification. The outcomes of this modeling approach reached the score of area under the curve of the receiver operating characteristics of 94.2 % for the polyps and 87.4 % for the nodules, resulting in an average gain of 5 % to 30 % over ten existing state-of-the-art lesion classification methods. The gains by modeling tissue elasticity for ML differentiation of lesions are striking, indicating the great potential of exploring the modeling strategy to other tissue properties for ML differentiation of lesions.
Low Dose CT Image Denoising Using a Generative Adversarial Network with Wasserstein Distance and Perceptual Loss
Apr 24, 2018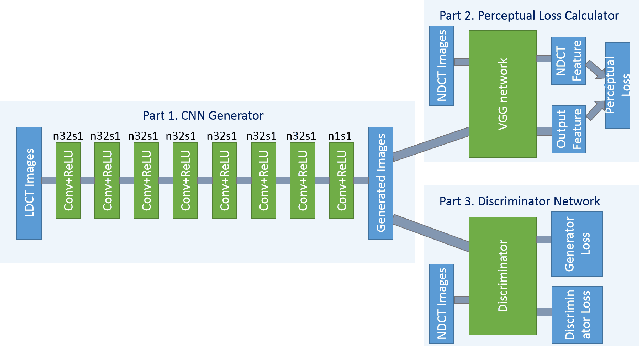
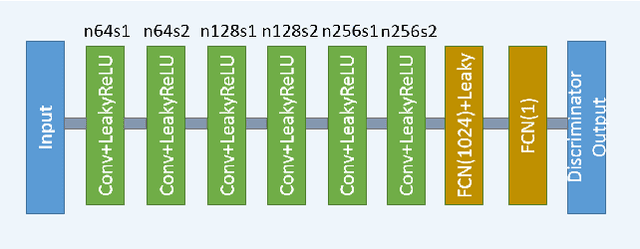
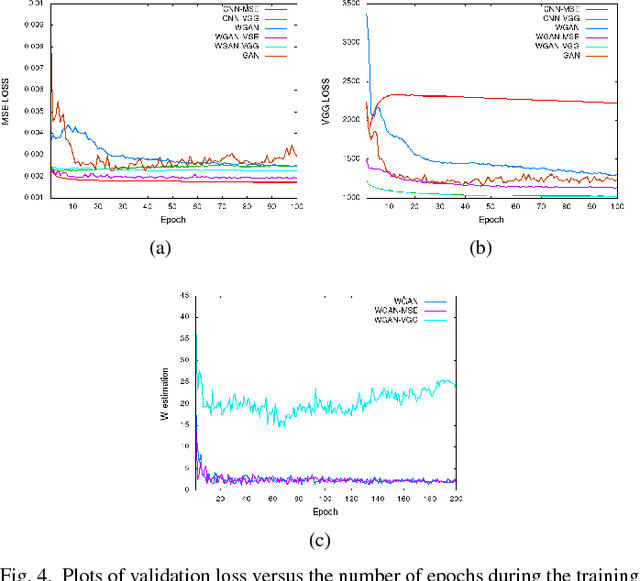
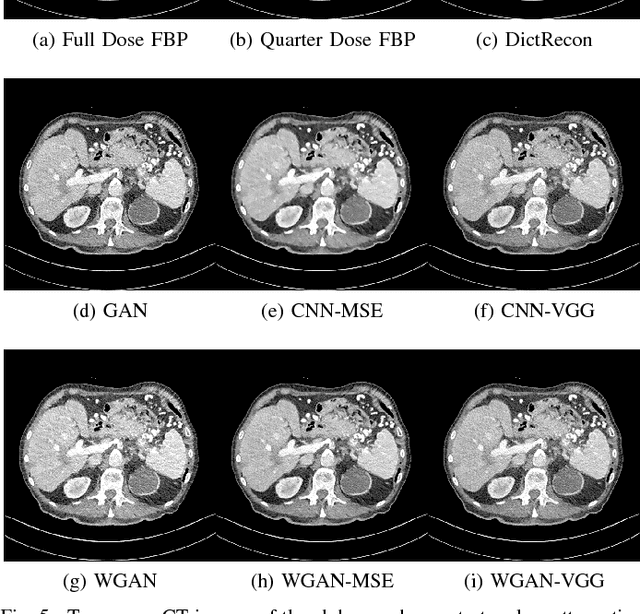
Abstract:In this paper, we introduce a new CT image denoising method based on the generative adversarial network (GAN) with Wasserstein distance and perceptual similarity. The Wasserstein distance is a key concept of the optimal transform theory, and promises to improve the performance of the GAN. The perceptual loss compares the perceptual features of a denoised output against those of the ground truth in an established feature space, while the GAN helps migrate the data noise distribution from strong to weak. Therefore, our proposed method transfers our knowledge of visual perception to the image denoising task, is capable of not only reducing the image noise level but also keeping the critical information at the same time. Promising results have been obtained in our experiments with clinical CT images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge