Weifang Zhu
AI-based Automatic Segmentation of Prostate on Multi-modality Images: A Review
Jul 09, 2024



Abstract:Prostate cancer represents a major threat to health. Early detection is vital in reducing the mortality rate among prostate cancer patients. One approach involves using multi-modality (CT, MRI, US, etc.) computer-aided diagnosis (CAD) systems for the prostate region. However, prostate segmentation is challenging due to imperfections in the images and the prostate's complex tissue structure. The advent of precision medicine and a significant increase in clinical capacity have spurred the need for various data-driven tasks in the field of medical imaging. Recently, numerous machine learning and data mining tools have been integrated into various medical areas, including image segmentation. This article proposes a new classification method that differentiates supervision types, either in number or kind, during the training phase. Subsequently, we conducted a survey on artificial intelligence (AI)-based automatic prostate segmentation methods, examining the advantages and limitations of each. Additionally, we introduce variants of evaluation metrics for the verification and performance assessment of the segmentation method and summarize the current challenges. Finally, future research directions and development trends are discussed, reflecting the outcomes of our literature survey, suggesting high-precision detection and treatment of prostate cancer as a promising avenue.
Uncertainty-inspired Open Set Learning for Retinal Anomaly Identification
Apr 08, 2023
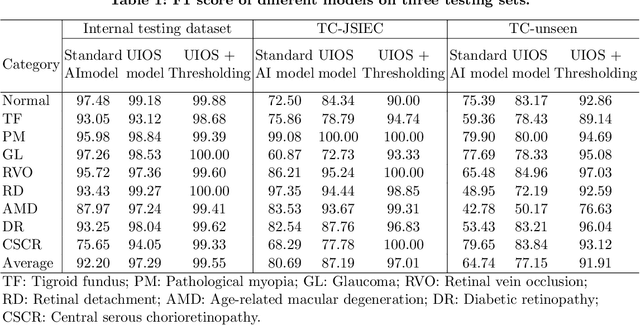


Abstract:Failure to recognize samples from the classes unseen during training is a major limit of artificial intelligence (AI) in real-world implementation of retinal anomaly classification. To resolve this obstacle, we propose an uncertainty-inspired open-set (UIOS) model which was trained with fundus images of 9 common retinal conditions. Besides the probability of each category, UIOS also calculates an uncertainty score to express its confidence. Our UIOS model with thresholding strategy achieved an F1 score of 99.55%, 97.01% and 91.91% for the internal testing set, external testing set and non-typical testing set, respectively, compared to the F1 score of 92.20%, 80.69% and 64.74% by the standard AI model. Furthermore, UIOS correctly predicted high uncertainty scores, which prompted the need for a manual check, in the datasets of rare retinal diseases, low-quality fundus images, and non-fundus images. This work provides a robust method for real-world screening of retinal anomalies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge