Tucker Netherton
Virtual Dosimetrists: A Radiotherapy Training "Flight Simulator"
May 14, 2025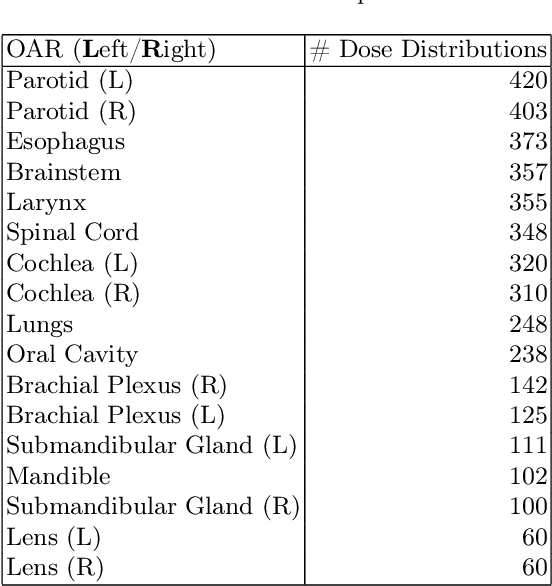
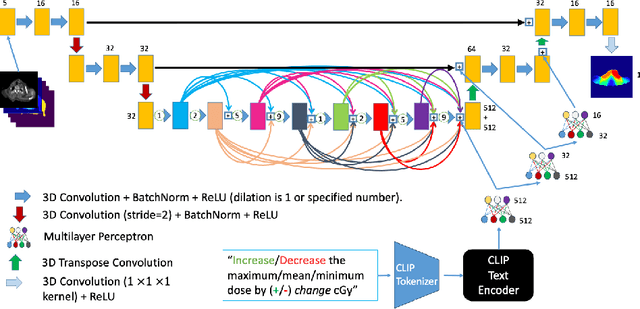
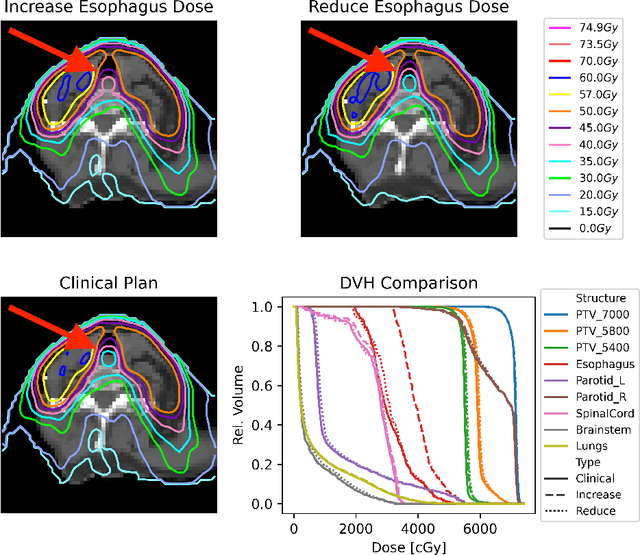
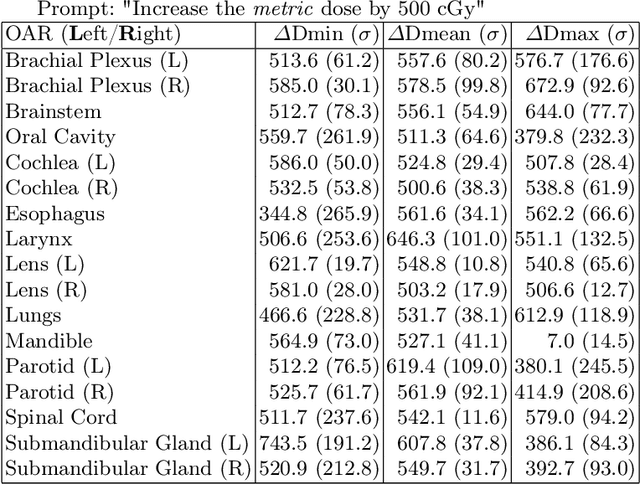
Abstract:Effective education in radiotherapy plan quality review requires a robust, regularly updated set of examples and the flexibility to demonstrate multiple possible planning approaches and their consequences. However, the current clinic-based paradigm does not support these needs. To address this, we have developed 'Virtual Dosimetrist' models that can both generate training examples of suboptimal treatment plans and then allow trainees to improve the plan quality through simple natural language prompts, as if communicating with a dosimetrist. The dose generation and modification process is accurate, rapid, and requires only modest resources. This work is the first to combine dose distribution prediction with natural language processing; providing a robust pipeline for both generating suboptimal training plans and allowing trainees to practice their critical plan review and improvement skills that addresses the challenges of the current clinic-based paradigm.
DIFR3CT: Latent Diffusion for Probabilistic 3D CT Reconstruction from Few Planar X-Rays
Aug 27, 2024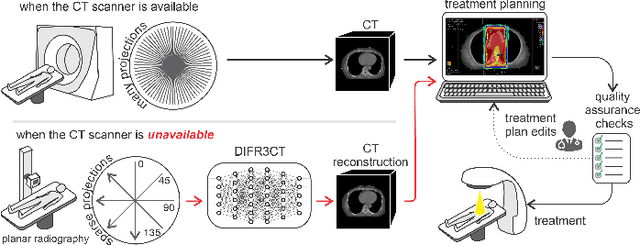
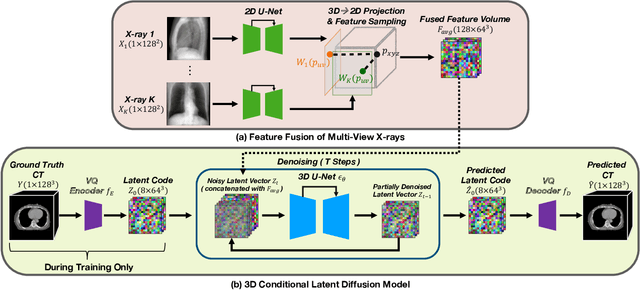
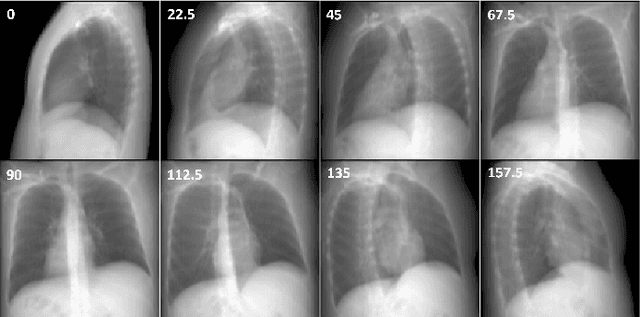
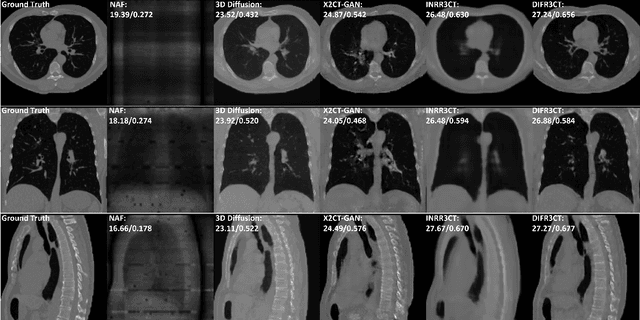
Abstract:Computed Tomography (CT) scans are the standard-of-care for the visualization and diagnosis of many clinical ailments, and are needed for the treatment planning of external beam radiotherapy. Unfortunately, the availability of CT scanners in low- and mid-resource settings is highly variable. Planar x-ray radiography units, in comparison, are far more prevalent, but can only provide limited 2D observations of the 3D anatomy. In this work we propose DIFR3CT, a 3D latent diffusion model, that can generate a distribution of plausible CT volumes from one or few (<10) planar x-ray observations. DIFR3CT works by fusing 2D features from each x-ray into a joint 3D space, and performing diffusion conditioned on these fused features in a low-dimensional latent space. We conduct extensive experiments demonstrating that DIFR3CT is better than recent sparse CT reconstruction baselines in terms of standard pixel-level (PSNR, SSIM) on both the public LIDC and in-house post-mastectomy CT datasets. We also show that DIFR3CT supports uncertainty quantification via Monte Carlo sampling, which provides an opportunity to measure reconstruction reliability. Finally, we perform a preliminary pilot study evaluating DIFR3CT for automated breast radiotherapy contouring and planning -- and demonstrate promising feasibility. Our code is available at https://github.com/yransun/DIFR3CT.
MIST: A Simple and Scalable End-To-End 3D Medical Imaging Segmentation Framework
Jul 31, 2024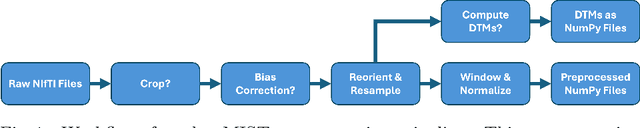
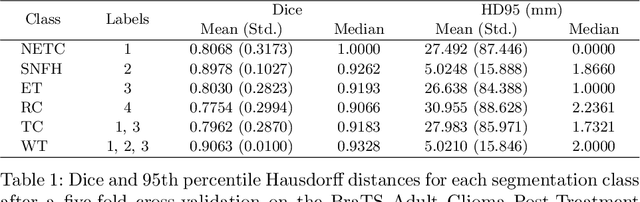
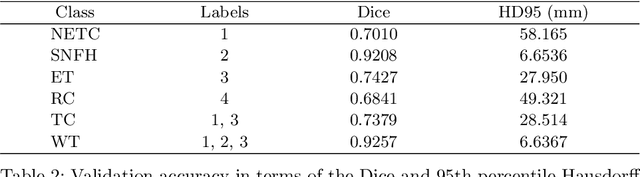
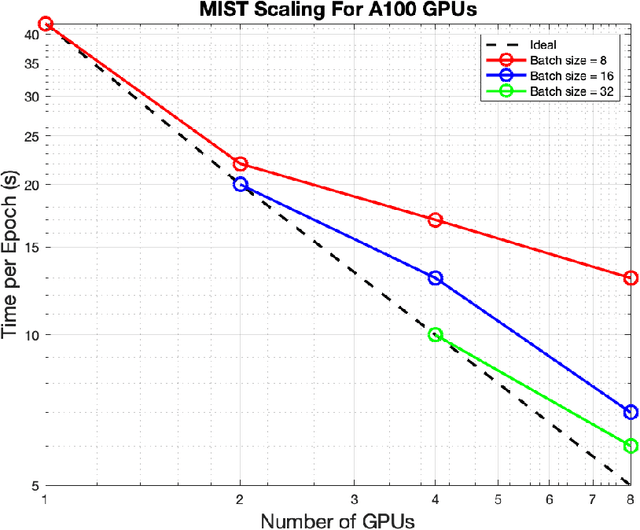
Abstract:Medical imaging segmentation is a highly active area of research, with deep learning-based methods achieving state-of-the-art results in several benchmarks. However, the lack of standardized tools for training, testing, and evaluating new methods makes the comparison of methods difficult. To address this, we introduce the Medical Imaging Segmentation Toolkit (MIST), a simple, modular, and end-to-end medical imaging segmentation framework designed to facilitate consistent training, testing, and evaluation of deep learning-based medical imaging segmentation methods. MIST standardizes data analysis, preprocessing, and evaluation pipelines, accommodating multiple architectures and loss functions. This standardization ensures reproducible and fair comparisons across different methods. We detail MIST's data format requirements, pipelines, and auxiliary features and demonstrate its efficacy using the BraTS Adult Glioma Post-Treatment Challenge dataset. Our results highlight MIST's ability to produce accurate segmentation masks and its scalability across multiple GPUs, showcasing its potential as a powerful tool for future medical imaging research and development.
CT Reconstruction from Few Planar X-rays with Application towards Low-resource Radiotherapy
Aug 04, 2023Abstract:CT scans are the standard-of-care for many clinical ailments, and are needed for treatments like external beam radiotherapy. Unfortunately, CT scanners are rare in low and mid-resource settings due to their costs. Planar X-ray radiography units, in comparison, are far more prevalent, but can only provide limited 2D observations of the 3D anatomy. In this work, we propose a method to generate CT volumes from few (<5) planar X-ray observations using a prior data distribution, and perform the first evaluation of such a reconstruction algorithm for a clinical application: radiotherapy planning. We propose a deep generative model, building on advances in neural implicit representations to synthesize volumetric CT scans from few input planar X-ray images at different angles. To focus the generation task on clinically-relevant features, our model can also leverage anatomical guidance during training (via segmentation masks). We generated 2-field opposed, palliative radiotherapy plans on thoracic CTs reconstructed by our method, and found that isocenter radiation dose on reconstructed scans have <1% error with respect to the dose calculated on clinically acquired CTs using <=4 X-ray views. In addition, our method is better than recent sparse CT reconstruction baselines in terms of standard pixel and structure-level metrics (PSNR, SSIM, Dice score) on the public LIDC lung CT dataset. Code is available at: https://github.com/wanderinrain/Xray2CT.
Automated WBRT Treatment Planning via Deep Learning Auto-Contouring and Customizable Landmark-Based Field Aperture Design
May 24, 2022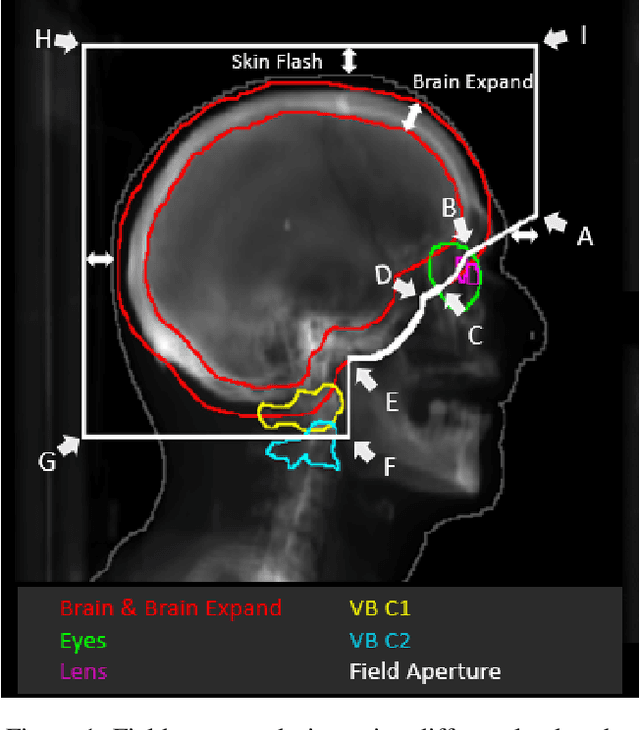


Abstract:In this work, we developed and evaluated a novel pipeline consisting of two landmark-based field aperture generation approaches for WBRT treatment planning; they are fully automated and customizable. The automation pipeline is beneficial for both clinicians and patients, where we can reduce clinician workload and reduce treatment planning time. The customizability of the field aperture design addresses different clinical requirements and allows the personalized design to become feasible. The performance results regarding quantitative and qualitative evaluations demonstrated that our plans were comparable with the original clinical plans. This technique has been deployed as part of a fully automated treatment planning tool for whole-brain cancer and could be translated to other treatment sites in the future.
OpenKBP-Opt: An international and reproducible evaluation of 76 knowledge-based planning pipelines
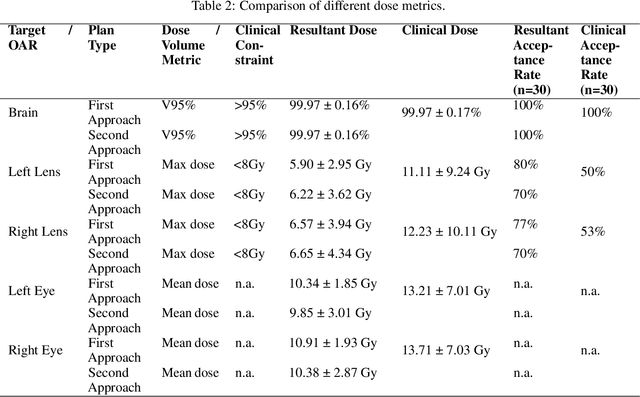
Feb 16, 2022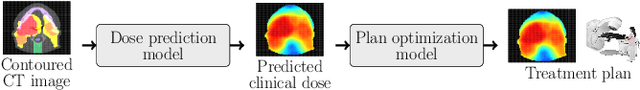

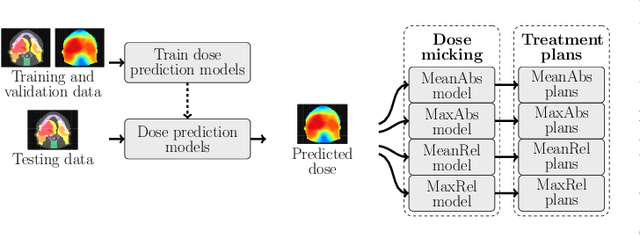
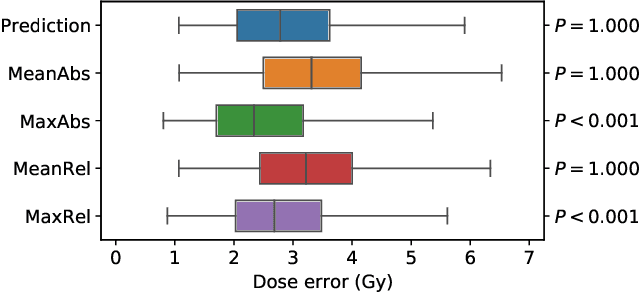
Abstract:We establish an open framework for developing plan optimization models for knowledge-based planning (KBP) in radiotherapy. Our framework includes reference plans for 100 patients with head-and-neck cancer and high-quality dose predictions from 19 KBP models that were developed by different research groups during the OpenKBP Grand Challenge. The dose predictions were input to four optimization models to form 76 unique KBP pipelines that generated 7600 plans. The predictions and plans were compared to the reference plans via: dose score, which is the average mean absolute voxel-by-voxel difference in dose a model achieved; the deviation in dose-volume histogram (DVH) criterion; and the frequency of clinical planning criteria satisfaction. We also performed a theoretical investigation to justify our dose mimicking models. The range in rank order correlation of the dose score between predictions and their KBP pipelines was 0.50 to 0.62, which indicates that the quality of the predictions is generally positively correlated with the quality of the plans. Additionally, compared to the input predictions, the KBP-generated plans performed significantly better (P<0.05; one-sided Wilcoxon test) on 18 of 23 DVH criteria. Similarly, each optimization model generated plans that satisfied a higher percentage of criteria than the reference plans. Lastly, our theoretical investigation demonstrated that the dose mimicking models generated plans that are also optimal for a conventional planning model. This was the largest international effort to date for evaluating the combination of KBP prediction and optimization models. In the interest of reproducibility, our data and code is freely available at https://github.com/ababier/open-kbp-opt.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge