Tobias Kober
1 and 3 and 4, §
Fast refacing of MR images with a generative neural network lowers re-identification risk and preserves volumetric consistency
May 26, 2023Abstract:With the rise of open data, identifiability of individuals based on 3D renderings obtained from routine structural magnetic resonance imaging (MRI) scans of the head has become a growing privacy concern. To protect subject privacy, several algorithms have been developed to de-identify imaging data using blurring, defacing or refacing. Completely removing facial structures provides the best re-identification protection but can significantly impact post-processing steps, like brain morphometry. As an alternative, refacing methods that replace individual facial structures with generic templates have a lower effect on the geometry and intensity distribution of original scans, and are able to provide more consistent post-processing results by the price of higher re-identification risk and computational complexity. In the current study, we propose a novel method for anonymised face generation for defaced 3D T1-weighted scans based on a 3D conditional generative adversarial network. To evaluate the performance of the proposed de-identification tool, a comparative study was conducted between several existing defacing and refacing tools, with two different segmentation algorithms (FAST and Morphobox). The aim was to evaluate (i) impact on brain morphometry reproducibility, (ii) re-identification risk, (iii) balance between (i) and (ii), and (iv) the processing time. The proposed method takes 9 seconds for face generation and is suitable for recovering consistent post-processing results after defacing.
Validation and Generalizability of Self-Supervised Image Reconstruction Methods for Undersampled MRI
Jan 29, 2022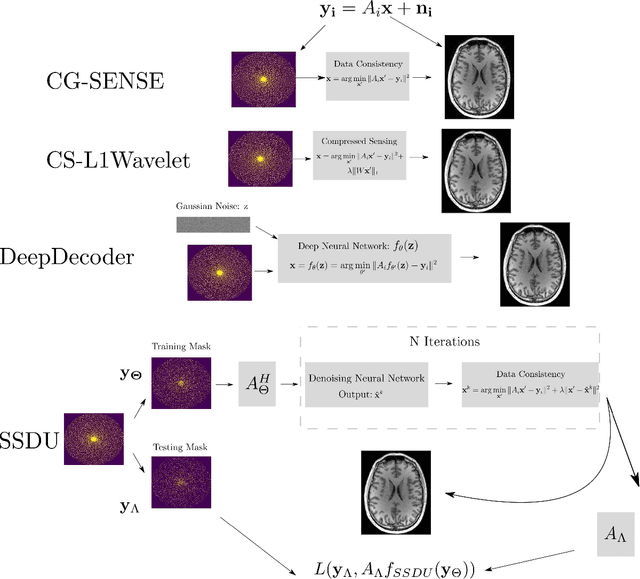
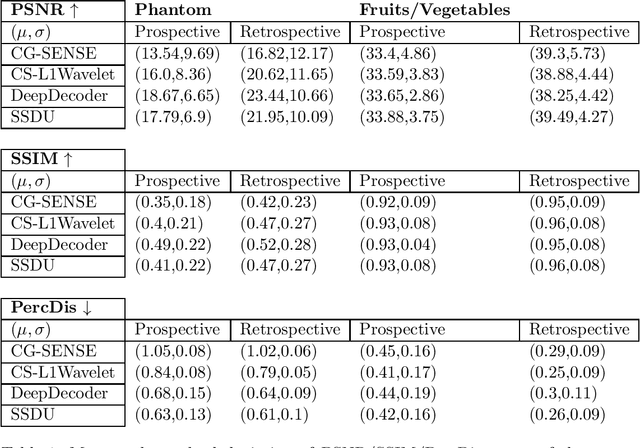
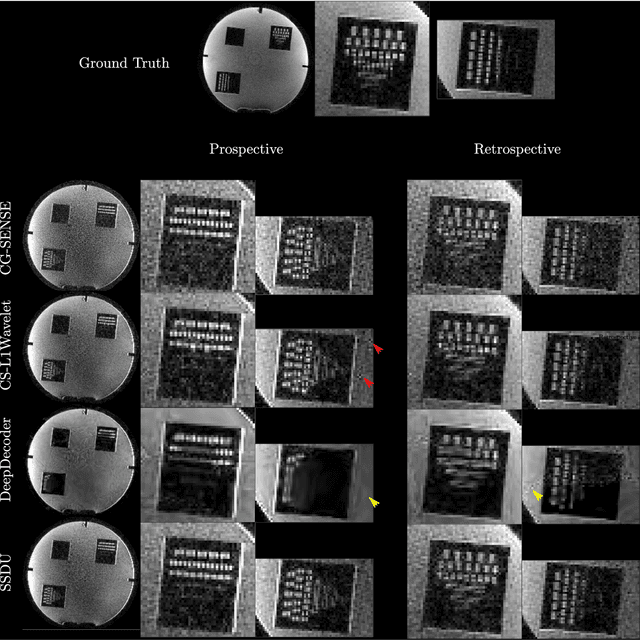
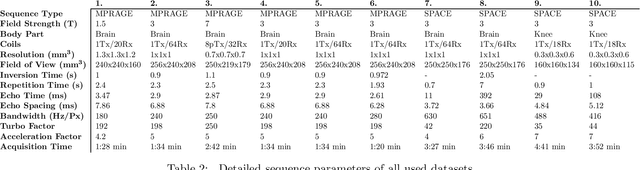
Abstract:Purpose: To investigate aspects of the validation of self-supervised algorithms for reconstruction of undersampled MR images: quantitative evaluation of prospective reconstructions, potential differences between prospective and retrospective reconstructions, suitability of commonly used quantitative metrics, and generalizability. Theory and Methods: Two self-supervised algorithms based on self-supervised denoising and neural network image priors were investigated. These methods are compared to a least squares fitting and a compressed sensing reconstruction using in-vivo and phantom data. Their generalizability was tested with prospectively under-sampled data from experimental conditions different to the training. Results: Prospective reconstructions can exhibit significant distortion relative to retrospective reconstructions/ground truth. Pixel-wise quantitative metrics may not capture differences in perceptual quality accurately, in contrast to a perceptual metric. All methods showed potential for generalization; generalizability is more affected by changes in anatomy/contrast than other changes. No-reference image metrics correspond well with human rating of image quality for studying generalizability. Compressed Sensing and learned denoising perform similarly well on all data. Conclusion: Self-supervised methods show promising results for accelerating image reconstruction in clinical routines. Nonetheless, more work is required to investigate standardized methods to validate reconstruction algorithms for future clinical use.
Cortical lesions, central vein sign, and paramagnetic rim lesions in multiple sclerosis: emerging machine learning techniques and future avenues
Jan 19, 2022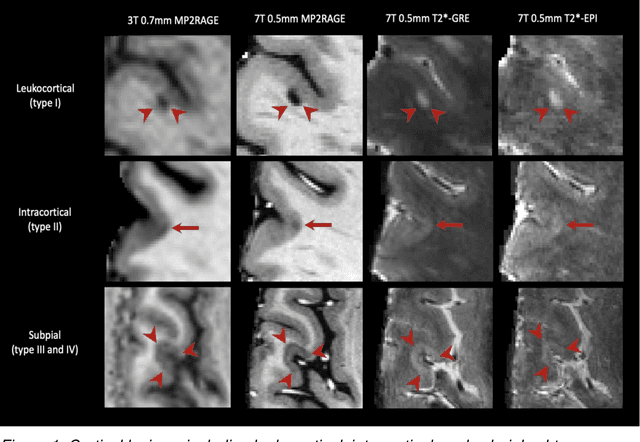
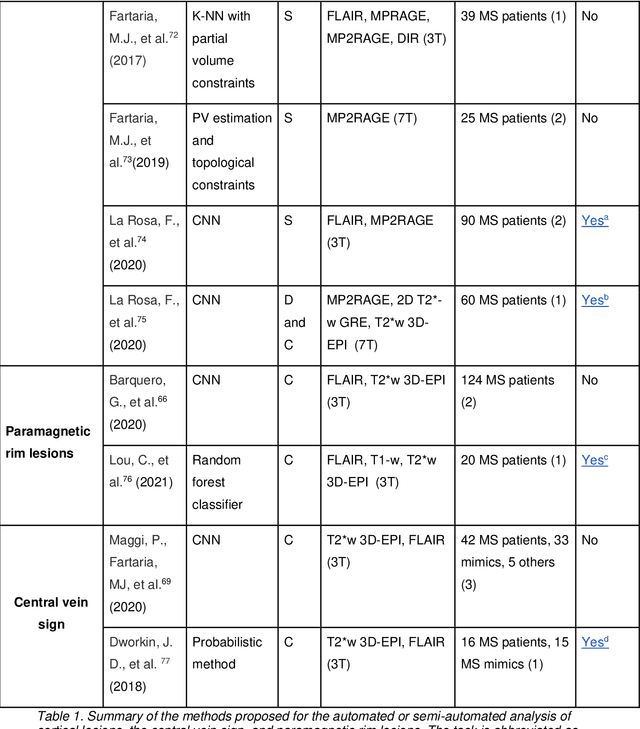
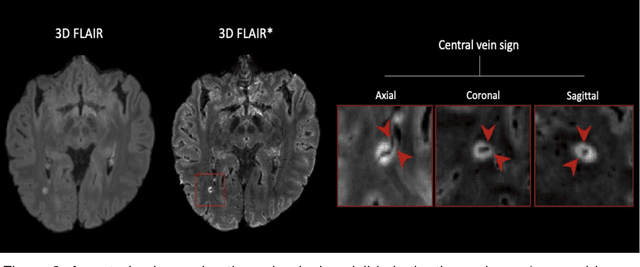
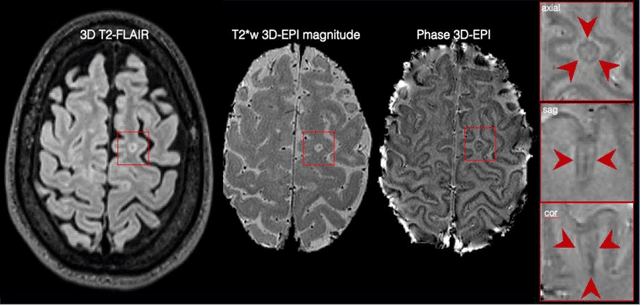
Abstract:The current multiple sclerosis (MS) diagnostic criteria lack specificity, and this may lead to misdiagnosis, which remains an issue in present-day clinical practice. In addition, conventional biomarkers only moderately correlate with MS disease progression. Recently, advanced MS lesional imaging biomarkers such as cortical lesions (CL), the central vein sign (CVS), and paramagnetic rim lesions (PRL), visible in specialized magnetic resonance imaging (MRI) sequences, have shown higher specificity in differential diagnosis. Moreover, studies have shown that CL and PRL are potential prognostic biomarkers, the former correlating with cognitive impairments and the latter with early disability progression. As machine learning-based methods have achieved extraordinary performance in the assessment of conventional imaging biomarkers, such as white matter lesion segmentation, several automated or semi-automated methods have been proposed for CL, CVS, and PRL as well. In the present review, we first introduce these advanced MS imaging biomarkers and their imaging methods. Subsequently, we describe the corresponding machine learning-based methods that were used to tackle these clinical questions, putting them into context with respect to the challenges they are still facing, including non-standardized MRI protocols, limited datasets, and moderate inter-rater variability. We conclude by presenting the current limitations that prevent their broader deployment and suggesting future research directions.
FaBiAN: A Fetal Brain magnetic resonance Acquisition Numerical phantom
Sep 06, 2021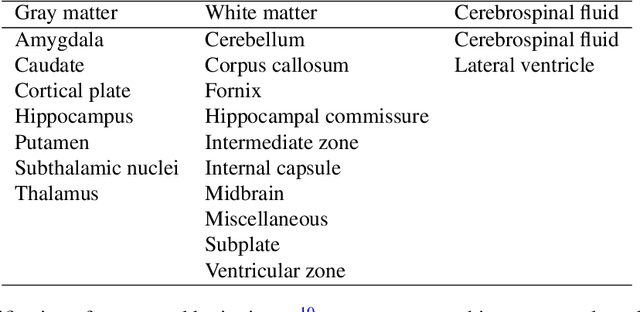
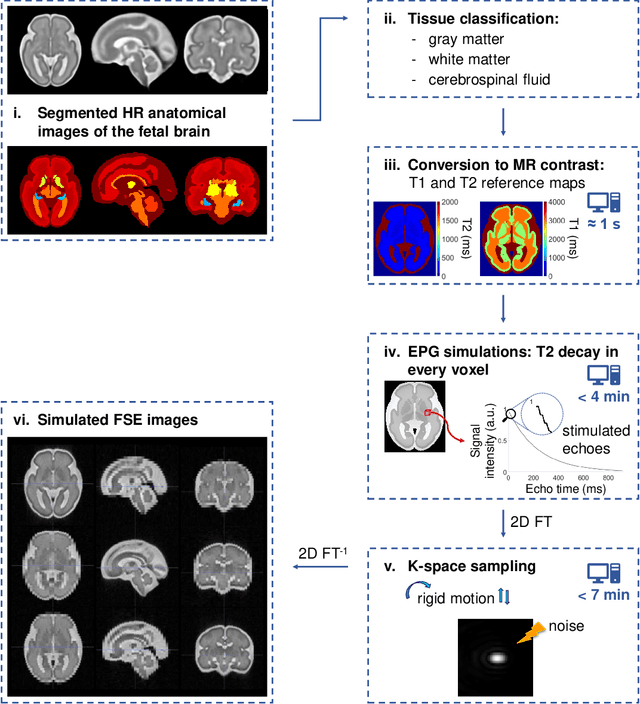
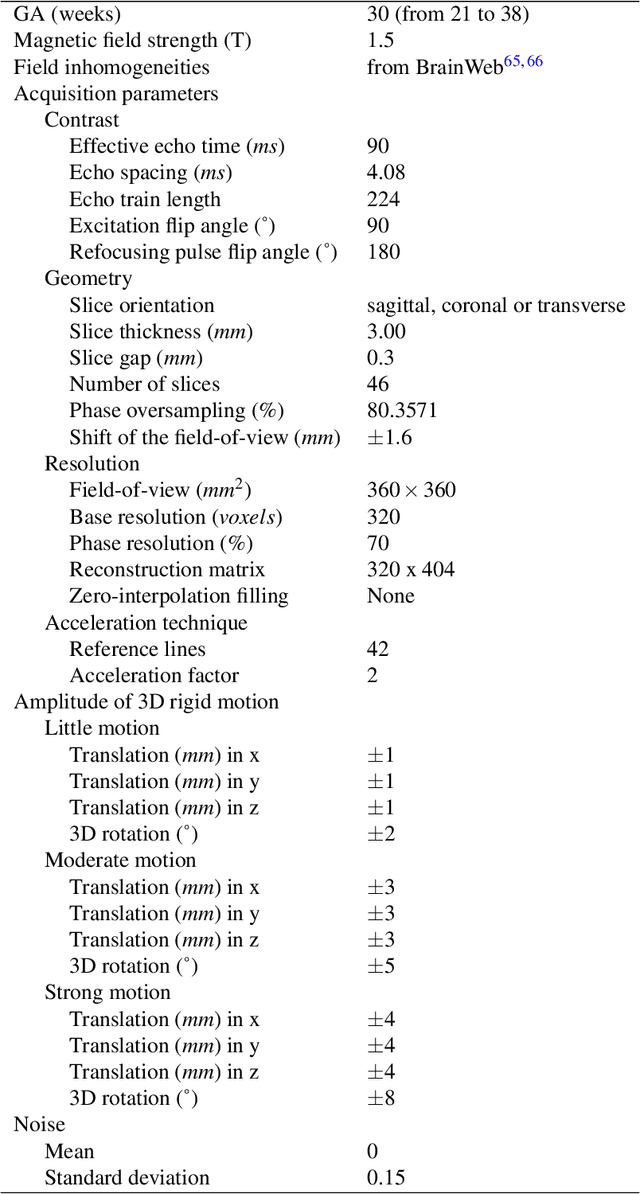
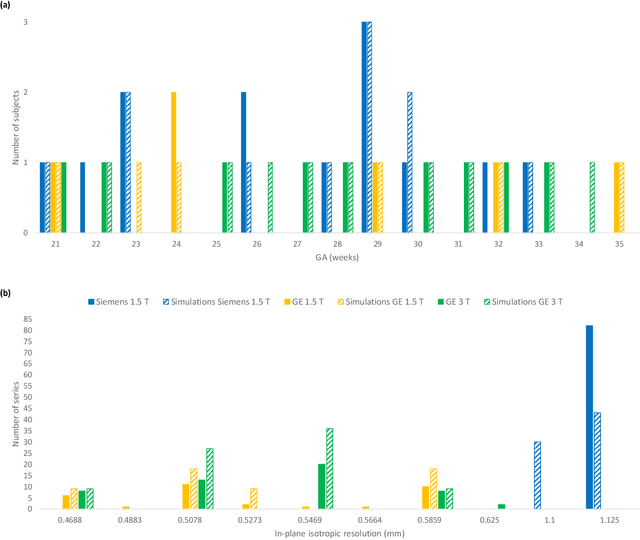
Abstract:Accurate characterization of in utero human brain maturation is critical as it involves complex and interconnected structural and functional processes that may influence health later in life. Magnetic resonance imaging is a powerful tool to investigate equivocal neurological patterns during fetal development. However, the number of acquisitions of satisfactory quality available in this cohort of sensitive subjects remains scarce, thus hindering the validation of advanced image processing techniques. Numerical phantoms can mitigate these limitations by providing a controlled environment with a known ground truth. In this work, we present FaBiAN, an open-source Fetal Brain magnetic resonance Acquisition Numerical phantom that simulates clinical T2-weighted fast spin echo sequences of the fetal brain. This unique tool is based on a general, flexible and realistic setup that includes stochastic fetal movements, thus providing images of the fetal brain throughout maturation comparable to clinical acquisitions. We demonstrate its value to evaluate the robustness and optimize the accuracy of an algorithm for super-resolution fetal brain magnetic resonance imaging from simulated motion-corrupted 2D low-resolution series as compared to a synthetic high-resolution reference volume. We also show that the images generated can complement clinical datasets to support data-intensive deep learning methods for fetal brain tissue segmentation.
Multi-compartment diffusion MRI, T2 relaxometry and myelin water imaging as neuroimaging descriptors for anomalous tissue detection
Apr 15, 2021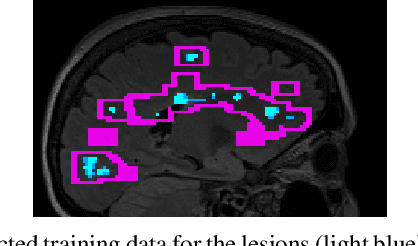
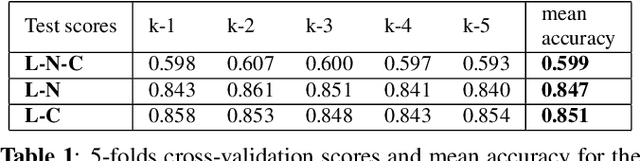
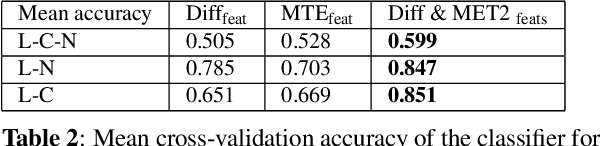
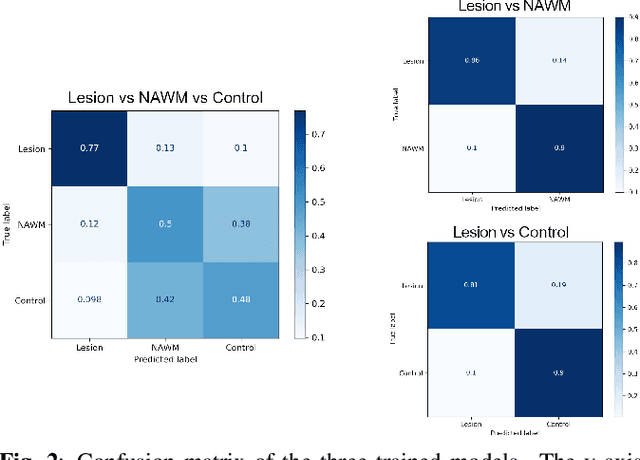
Abstract:Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease characterized by diffuse and focal areas of tissue loss. Conventional MRI techniques such as T1-weighted and T2-weighted scans are generally used in the diagnosis and prognosis of the disease. Yet, these methods are limited by the lack of specificity between lesions, their perilesional area and non-lesional tissue. Alternative MRI techniques exhibit a higher level of sensitivity to focal and diffuse MS pathology than conventional MRI acquisitions. However, they still suffer from limited specificity when considered alone. In this work, we have combined tissue microstructure information derived from multicompartment diffusion MRI and T2 relaxometry models to explore the voxel-based prediction power of a machine learning model in a cohort of MS patients and healthy controls. Our results show that the combination of multi-modal features, together with a boosting enhanced decision-tree based classifier, which combines a set of weak classifiers to form a strong classifier via a voting mechanism, is able to utilise the complementary information for the classification of abnormal tissue.
Shallow vs deep learning architectures for white matter lesion segmentation in the early stages of multiple sclerosis
Sep 10, 2018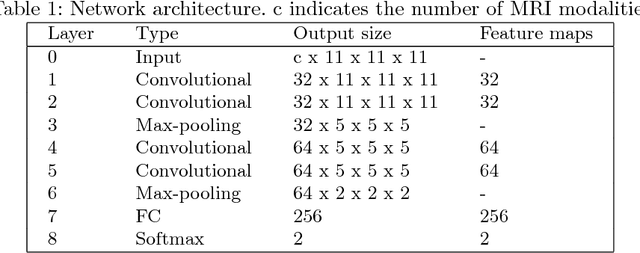
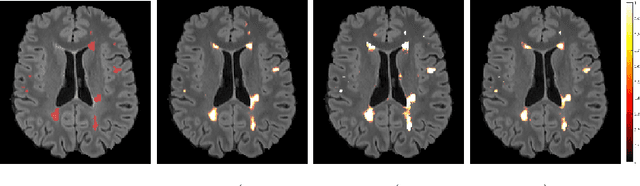
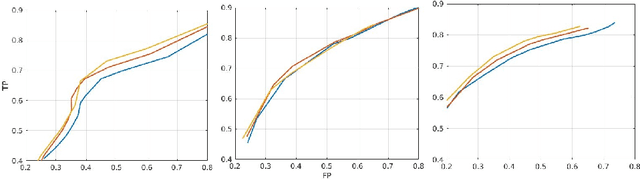
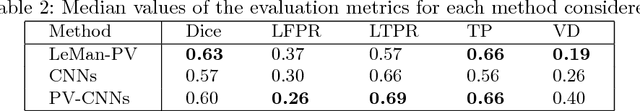
Abstract:In this work, we present a comparison of a shallow and a deep learning architecture for the automated segmentation of white matter lesions in MR images of multiple sclerosis patients. In particular, we train and test both methods on early stage disease patients, to verify their performance in challenging conditions, more similar to a clinical setting than what is typically provided in multiple sclerosis segmentation challenges. Furthermore, we evaluate a prototype naive combination of the two methods, which refines the final segmentation. All methods were trained on 32 patients, and the evaluation was performed on a pure test set of 73 cases. Results show low lesion-wise false positives (30%) for the deep learning architecture, whereas the shallow architecture yields the best Dice coefficient (63%) and volume difference (19%). Combining both shallow and deep architectures further improves the lesion-wise metrics (69% and 26% lesion-wise true and false positive rate, respectively).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge